Discovery of the proton. Proton-electronic model of the nucleus
Fig.131
Unit of exposure dose rate in SI coulomb per kilogram per second.
Off-system unit - x-ray per hour. 1 R ç
h = 7.17 C ç
kg s. Exposure dose rate 1 Р ç
h corresponds to the absorbed dose rate 8.77 10 -3 Gy ç
3600 s \u003d 2.44 10 - -6 Gy ç
With.
9. Biological effect of radioactive radiation was discovered and began to be studied already in the first years after the discovery of radioactivity. With the beginning of the use of atomic weapons in 1945, and then the peaceful use of atomic energy, these studies became especially intensive. The main features of the biological action of radiation are as follows:
a.Profound disturbances in vital functions are caused by negligible amounts of energy. So, the energy absorbed by the body of a mammal or a person when irradiated with a lethal dose, if it were converted into heat, would lead to heating of the body by only 0.001 ° C.
b.Radiation damages the hereditary apparatus, its effect affects not only the irradiated individual, but also his subsequent offspring.
in. Radiation damage is latent (hidden) in nature . It does not appear immediately, but after some time.
The interaction of radiation with a biological object is characterized by equivalent dose
D e = kD, where D- absorbed dose k- so-called quality factor. Value k the more dangerous this species radiation for a living organism. For β
- and
g-rays k= 1, for slow neutrons k= 3, for fast neutrons and protons k= 10, for nuclear fission fragments k = 20.
Unit of equivalent dose in SI sievert(Sv), 1 Sv = 1 J ç kg.
Until recently, the equivalent dose was measured in terms of rems. Baer is an abbreviation for the phrase biological equivalent of rad. 1 rem = 0.01 Sv, 1 Sv = 100 rem.
§eighteen. The problem of the structure of the atomic nucleus
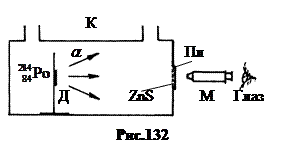 1. Discovery of the proton .
In 1919 Ernst Rutherford pioneered the artificial conversion of nitrogen nuclei. The installation diagram is shown in Fig.132. The alpha-active preparation of polonium was applied to the holder D inside the cuvette K. Scintillations a-particles could be observed on a glass plate Pl with ZnS powder through a microscope M. Alpha particles have an energy of 7.58 MeV and a free path in air at normal pressure of about 7 cm. gas pressure in the cell a-particles could reach the Pl plate and cause scintillations on it. At normal and elevated pressure a-particles did not reach the Pl plate.
1. Discovery of the proton .
In 1919 Ernst Rutherford pioneered the artificial conversion of nitrogen nuclei. The installation diagram is shown in Fig.132. The alpha-active preparation of polonium was applied to the holder D inside the cuvette K. Scintillations a-particles could be observed on a glass plate Pl with ZnS powder through a microscope M. Alpha particles have an energy of 7.58 MeV and a free path in air at normal pressure of about 7 cm. gas pressure in the cell a-particles could reach the Pl plate and cause scintillations on it. At normal and elevated pressure a-particles did not reach the Pl plate.
The cuvette was filled with different gases, after which scintillations were observed a-particles at low pressures and their disappearance with increasing pressure.
When the cuvette was filled with nitrogen N 2, scintillations were observed on the Pl plate even at pressures higher than the limiting one. Because the a-particles could not reach the phosphor, it remained to make the assumption that in the process of interaction a-particles with nitrogen nuclei were born some other ionizing particles.
The superposition of electric and magnetic fields made it possible to establish that a new particle being born has positive charge, equal in absolute value to the electron charge, and a mass approximately equal to the mass of hydrogen. Obviously, this is the nucleus of a hydrogen atom, as a-particle - the nucleus of a helium atom.
Back in the 1910s, the idea was put forward that the nuclei of all chemical elements consist of hydrogen nuclei - that is, the nuclei of the simplest, first element in the table. Therefore, Rutherford called the new particle "proton", from Greek. protos- the first. (Even earlier, in 1815, a similar idea regarding the composition of chemical elements was expressed by the chemist William Prout).
Patrick Blackett continued in 1925 experiments with nitrogen using a cloud chamber improved by him (see p. 132). He automated the shooting of foggy tracks of ionizing particles with two cameras simultaneously from different directions. This made it possible to reconstruct the spatial form of particle trajectories. After reviewing 23,000 photographs, he determined that a-particle in the collision is absorbed by the nitrogen nucleus, after which this new nucleus ejects a proton. Taking into account the conservation of charge and mass, a nuclear reaction can be written as follows: ![]() Rutherford, 1919 Discovery of the proton (18.1)
Rutherford, 1919 Discovery of the proton (18.1)
The proton energy in Rutherford's experiments was about 6 MeV, and the path length was 28 cm.
2. Properties of the proton. Proton in a free state - stable elementary particle , the nucleus of a hydrogen atom. In nuclear reactions, it is often denoted by the symbol . proton mass m p almost 2000 times the mass of an electron me, m p = 1836me\u003d 1.67239 10 -27 kg.
Spin, that is own angular momentum a proton is the same as an electron. Its projection onto the physical axis can take only two values, L sz =±ћ/ 2. Spin quantum number s a proton, like an electron, is a half-integer, . Therefore, the system of protons, like the system of electrons, is described Fermi-Dirac statistics.
Magnetic spin moment of the proton. As already mentioned in Section 2, in Bohr's theory of the hydrogen atom, the orbital magnetic moment of an electron in the lowest energy state ( n= 1) is equal to J ç Tl (see f. 2.18). At a higher energy level, the magnetic moment in n times more where n– level number, M n = n M one . The minimum value of the magnetic moment is called Bohr magneton. This is the minimum magnetic moment in the physics of the electron shells of atoms. Therefore, the Bohr magneton is used as a unit of measurement for the magnetic moments of electrons.
From the experiments of Stern and Gerlach on the splitting of atomic beams in an inhomogeneous magnetic field, it followed that the spin magnetic moment of an electron is equal to the Bohr magneton. Therefore, after the discovery of the proton, it was natural to assume that the spin magnetic moment of the proton is determined by the Bohr magneton formula, in which instead of the electron mass me should stand proton mass m p. ![]() J ç
Tl. (18.2)
J ç
Tl. (18.2)
the value M i call nuclear magneton. It is 1836 times smaller than the Bohr magneton and is used as a unit of measurement for magnetic moments in nuclear physics.
But the measurements showed that the spin magnetic moment of the proton is 2.79 times greater than the nuclear magneton and is ![]() J ç
Tl. (18.3)
J ç
Tl. (18.3)
3. Nuclear reactions with proton emission observed later during the shelling a-particles of boron, fluorine, sodium, aluminum and phosphorus.
notice, that a-particles can effectively interact only with light nuclei. To overcome the electrical repulsion of the nucleus, a-particle must have kinetic energy E, not less than the potential energy required for the reaction of approach to the nucleus. ![]() (18.4)
(18.4)
From here you can find the maximum number Z element whose kernel is available to
a-particles with energy E. ![]() . (18.5)
. (18.5)
Core radius m. For a-particles with energy E≈ 10 MeV, we get
This is an overestimated number. As experiments show, effective interaction
a-particles with nuclei is real only for elements with Z≤ 20, that is, up to calcium.
4. Discovery of the neutron. By 1930, it turned out that some elements, for example, Be, Li, O 2, when fired a-particles do not emit protons. Therefore, the question arose: what happens when the shelled a-particles the nucleus does not emit a proton?
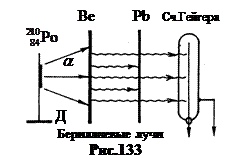 In 1930 Walter Bothe and Hans Becker set up an experiment, the scheme of which is shown in Fig. 133. On holder D was placed a- active drug. Its peculiarity is that having experienced a- the decay of the nucleus of polonium -210 turns into the nucleus of a stable isotope of lead. Therefore, the preparation of polonium-210 does not give any other radiation. Energy a-particles y is smaller than y, it is 5.25 MeV. But in order to penetrate the nuclei of the light beryllium chosen for the experiments, which has Z= 4, that was enough.
In 1930 Walter Bothe and Hans Becker set up an experiment, the scheme of which is shown in Fig. 133. On holder D was placed a- active drug. Its peculiarity is that having experienced a- the decay of the nucleus of polonium -210 turns into the nucleus of a stable isotope of lead. Therefore, the preparation of polonium-210 does not give any other radiation. Energy a-particles y is smaller than y, it is 5.25 MeV. But in order to penetrate the nuclei of the light beryllium chosen for the experiments, which has Z= 4, that was enough.
It was feared that nuclei that do not emit protons emit other radiation that does not give flashes in zinc sulfide ZnS. Therefore, the zinc sulfide screen was replaced by a Geiger counter. Its action is based on the fact that a nuclear particle flying into the counter ionizes the gas in it. As a result, a current pulse appears in the counter circuit (for more details on the Geiger counter, see p. 130).
When irradiated a-particles of plates from beryllium, boron, lithium, as experience has shown, some kind of radiation arises, causing weak discharges of the Geiger counter. Beryllium gave particularly strong radiation. These beryllium the rays had a tremendous penetrating effect: a lead plate 2 cm thick reduced their intensity by only 14%.
Bothe and Becker suggested that beryllium rays are very hard g-quanta. By absorption in lead, it was found that their energy is 7 MeV. But energy a-particles was 5.25 MeV. Where did the energy boost come from?
In 1931, Irene and Frederic Joliot-Curie. Placing sheets of paraffin instead of lead plates, they found that protons were emitted from paraffin under the action of beryllium rays, the maximum range in the air of which was 26 cm. This range corresponded to a proton energy of 4.5 MeV.
Paraffins are saturated hydrocarbons with general formula C n H 2 n+2 . In them big number hydrogen atoms. The appearance of protons during the irradiation of paraffin with beryllium rays was interpreted by the Curies as a result of Compton scattering g-quanta on protons - the nuclei of hydrogen atoms. The recoil proton moves forward when scattered g the quantum is reflected back, θ
= π
. From here ![]() where m p is the mass of the proton, λ
0 is the wavelength of beryllium g-quanta. Energy E of the knocked out proton should be equal to the loss of energy g-quantum, (18.6)
where m p is the mass of the proton, λ
0 is the wavelength of beryllium g-quanta. Energy E of the knocked out proton should be equal to the loss of energy g-quantum, (18.6)
Let's assume that 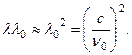 Then
Then 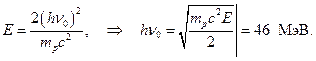
So, the energy of beryllium g-quanta, calculated from absorption in lead, 7 MeV, from the energy of protons knocked out of paraffin - 46-48 MeV. Moreover, it turns out that α -particles with an energy of 5.25 MeV knock out g-quantum with energy 46-48 MeV!
In 1932, he was included in the study of the nature of beryllium rays. James Chadwick. The scheme of its installation was practically no different from the scheme of Bothe and Becker, only instead of the Geiger counter, he, like the Curie, used an ionization chamber. Chadwick investigated the scattering of beryllium rays not only by paraffin, but also by other substances. Energy g-quanta measured from the energy of nitrogen recoil nuclei turned out to be equal to hν= 150 MeV.
So, the hypothesis according to which beryllium rays are g-quanta, led to contradictory results. Depending on the measurement method, the energy g-quanta was different and amounted to 7, 48, 150 MeV. This proved the incorrectness of this hypothesis.
In the same 1932, Chadwick came to the conclusion that beryllium radiation is flux of neutral particles which he called neutrons. The symbol for neutrons is . The nuclear reaction of neutron production can be written as follows:
![]() Neutron discovery reaction, 1930-32 .
(18.7)
Neutron discovery reaction, 1930-32 .
(18.7)
Chadwick also made the first measurements of the mass of the neutron. It turned out that the mass of the neutron is close to the mass of the proton. The exact value of the neutron mass was obtained from the mass balance of various nuclear reactions involving neutrons.
5. Properties of the neutron. Free neutron - unstable elementary particle, which decays into a proton, an electron, and an electron antineutrino. ![]() (18.8)
(18.8)
Mean neutron lifetime τ ≈ 16 minutes. The mass of a neutron is somewhat larger than the mass of a proton and is m n = 1838 me\u003d 1.6760 10 -27 kg. The neutron spin is ħç 2. Therefore, neutrons, like electrons and protons, are described Fermi-Dirac statistics.
The ratio of the spin magnetic moments of the proton and neutron is
M p cM n = - 3ç
2. "Minus" means that the directions of the intrinsic mechanical and magnetic moments of the neutron are opposite.
The high penetrating power of neutrons is due to their lack of electric charge. Neutrons practically do not interact with the electron shells of atoms and, unlike a-particles and protons do not repel from nuclei. Therefore, even at low energies, neutrons can come close to atomic nuclei and be captured by them.
To register fast neutrons, their elastic collisions with hydrogen nuclei are used. Due to the practical equality of the masses of the proton and neutron, during the elastic impact of a neutron with a stationary proton, most of the kinetic energy of the neutron is transferred to the latter. As a result, the neutron practically stops, and the proton moves in the same direction with an energy close to the initial energy of the neutron. Along the way, the proton produces intense ionization and therefore can be registered by an ionization chamber, a Geiger counter, or a cloud chamber.
After several successive collisions with atomic nuclei, fast neutrons give up their excess energy and subsequently perform a chaotic motion with thermal velocities. For such thermal neutrons, the method of registration described above by scattering on the nuclei of hydrogen atoms is unsuitable. In this case, nuclear reactions are used, in which a neutron, penetrating into the nucleus, leads to an escape from the latter. a particles of high energy. For example, ![]() (18.10)
(18.10)
6. The problem of the structure of the nucleus. By the beginning of the 30s of the XX century. The following structural elements of the atom were discovered: electron, 1897, Thomson; proton, 1919 Rutherford;
neutron, 1932 Chadwick. The discovery of radioactivity and the observation of the first nuclear reactions made the question urgent: how is the atomic nucleus arranged?
First of all, it became obvious that the nucleus of an atom cannot be represented as a ball made of some immovable nuclear bricks. The nucleus of an atom is very a small volume of space in which nuclear elements move. That is, it is a system of nuclear objects moving and interacting according to some yet unknown laws.
First of all, it was necessary to answer the question: what particles does the nucleus consist of. Historically, two options have been considered: proton-electron and proton-neutron nuclei.
a.Proton-electron nucleus. Before the discovery of the neutron in 1930 Paul Dirac analyzed Prout's idea that all chemical elements are composed of hydrogen. As applied to the problem of the structure of the nucleus, this idea boiled down to the fact that all the nuclei of elements consist of the nuclei of the hydrogen atom, that is, of protons. (The hydrogen isotope deuterium was discovered only 2 years later). But this means that the serial number of the element in the periodic table must be equal to its mass number. But there are no such elements in the table. Helium already has a serial number Z= 2, and the mass number BUT= 4. As if out of 4 protons in the nucleus, 2 are neutralized. It can be assumed that the helium nucleus contains 4 protons and 2 electrons. But in this case, there are contradictions with the Heisenberg uncertainty principle. Indeed, the uncertainty of the momentum in the nucleus, expressed from the uncertainty relation for the coordinate-momentum is: (18.11)
But the uncertainty of the coordinate Δ X cannot be greater than the core radius, at least Δ X≈ r 0 . From the experiments of Rutherford in 1909 r 0 ≈ 10 –15 m. The momentum of an electron in the nucleus cannot be less than the uncertainty of the momentum, ![]() and its minimum speed from the relativistic formula (18.12)
and its minimum speed from the relativistic formula (18.12)
Here me is the rest mass of the electron. After calculations, we get v = 0,99998c, where With is the speed of light. Calculated by the relativistic formula kinetic energy electron in the nucleus is 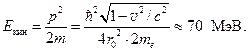
But the electrons emitted from the nucleus at β -decay, have an energy within 10 MeV. Such a striking discrepancy is very difficult to find a convincing explanation.
The second difficulty of the proton-electron model of the nucleus is called nitrogen catastrophe. Its essence is as follows.
From the hyperfine splitting of atomic spectra, it was possible to calculate the magnetic moment atomic nuclei. It turned out to be about 1000 times less than the spin magnetic moment of the electron. If there is an even number of electrons in the nucleus, then such a small magnetic moment of the nucleus can be explained by the fact that the electrons in the nucleus form pairs with opposite spins, so the magnetic moment of the nucleus is made up of the moments of protons. And the magnetic moment of the proton is 658 times less than the moment of the electron.
But in the nucleus of a nitrogen atom there must be 14 protons and 7 electrons. If 6 electrons form pairs with opposite spins, then one electron remains unpaired. This unpaired electron should provide the nitrogen nucleus with the same magnetic moment as it has, that is, 1000 times more than nuclei with an even number of electrons. But this is not. The magnetic moment of the nitrogen nucleus is of the same order as that of other nuclei.
b.Proton-neutron nucleus. Less than six months after the discovery of the neutron, almost simultaneously and independently of each other Dmitry Ivanenko and Werner Heisenberg suggested proton-neutron core composition. The proton-neutron model of the nucleus developed later is in excellent agreement with experiment and is now generally accepted. According to this model, the nucleus of an atom contains Z protons and A-Z neutrons. Here Z- the serial number of the element in the periodic table. Core charge q= Ze +, so the number Z called often charge number. the value BUT called mass number. This is an integer equal to the atomic mass of the element expressed in carbon units and rounded to the nearest whole number. The protons and neutrons in the nucleus are called nucleons(from lat. nucleus- nucleus). This emphasizes that the proton and neutron in the composition of the nucleus are one and the same particle in different states. Mass number BUT is the number of nucleons in the nucleus. Depending on the ratio between the numbers of protons and neutrons in nuclei, there are isotopes, isobars and isotones.
isotopes(from Greek isos - equal, topos - place) - nuclei containing the same number of protons, that is, having the same number Z, and a different number of neutrons. All isotopes are placed in one cell of the periodic table and are varieties of one chemical element. Isotopes differ in number N neutrons in the nucleus. For example, isotopes of hydrogen:
Protium, , nucleus-proton, Z = 1, A = 1, N = A–Z= 0, there are no neutrons.
Deuterium, , nucleus-deuteron, Z = 1, A = 2, N= 1, one neutron.
Tritium, , triton nucleus, Z = 1, A = 3, N= 2, two neutrons.
Helium isotopes:
, Z= 2 (two protons), A = 3, N= 1 (one neutron), stable.
, Z = 2, A = 4, N= 2 (two neutrons), stable.
, Z = 2, A = 6, N= 4 (four neutrons), unstable.
, Z = 2, A = 8, N= 6 (six neutrons), unstable.
The currently known elements have a number Z reaches approximately 105. This number of elements accounts for approximately 1500 known isotopes. Average per item
14 isotopes. Of which 1 ç
5 - stable and 4 ç
5 - unstable.
Isotopes of the same chemical element have the same chemical and almost identical physical properties. Therefore, the separation of isotopes is a complex physicochemical problem. Hydrogen isotopes differ most markedly.
isobars(from isos- and Greek baros- gravity) - nuclei with the same mass number BUT. For example, tritium and helium. They have 3 nucleons, but the ratio between protons and neutrons is different. tritium Z = 1, N= 2, for helium Z = 2, N = 1.
isotons are nuclei with the same number of neutrons. Like isobars, isotones are nuclei of different chemical elements. For example, nuclei and contain 3 neutrons.
The words isobars and isotones used much less frequently than the word isotopes.
1. Kernel sizes determined in three ways: by scattering on the nuclei of fast electrons, by studying the spectra mesoatoms and diffraction by neutron nuclei.
a. Scattering on the nuclei of fast electrons allows you to determine electric radius of the nucleus R email Electrons, whose energy must be at least 100 MeV, experience electromagnetic, but not nuclear, interaction with the nucleus. Therefore, based on their scattering, one can actually judge only the distribution of protons in the nucleus.
b. Spectra of mesoatoms , that is, atoms in which one of the electrons is replaced muon. Muon µ - elementary particle, its charge equal to the charge electron, and the mass is 207 times greater, m= 207me. Like the electron, the muon does not participate in nuclear interactions. The spectra of mesoatoms give more information about the structure of the nucleus than the spectra of ordinary atoms, since the muon, due to its greater mass, moves 207 times closer to the nucleus than the electron. The muon spends a relatively noticeable time even inside the nucleus.
in. Diffraction by neutron nuclei with an energy of the order of 20 MeV has the advantage that neutrons experience nuclear interaction with the core. The radius of this interaction is very small. Therefore, neutrons noticeably diffract on the nucleus, that is, they deviate from rectilinear motion, only flying very close to the nucleus and inside the nucleus itself. From the width of the diffraction maximum, which is formed by neutrons that have passed both outside and inside the nucleus, one can estimate both the size and the degree of transparency of the nucleus for neutrons.
The diffraction of neutrons by nuclei makes it possible to determine the radius of the region in which the nuclear forces of attraction act. In fact, this is the area in which the nucleons of the nucleus are concentrated. It follows from the experiments that the radius of the nucleus is proportional to the cube root of the number A of the nucleons contained in it, ![]() m. (19.1)
m. (19.1)
Let us calculate the concentration of nucleons in the nucleus. For this, the mass number A must be divided by the volume of the nucleus. .
Multiplying by the mass of one nucleon m p= 1.67·10 -27 kg, we obtain the average density ρ of the nuclear substance. .
The density of nuclear matter does not depend on the number of nucleons in the nucleus. It is the same in all nuclei and is a gigantic value. One cubic millimeter of such a substance would have a mass of 200,000 tons. That the density ρ of nuclear matter is constant, indicates that the nucleons in the nucleus in the sense of packing are similar to liquid molecules. The average volume per nucleon in a nucleus does not depend on the size of the nucleus, just as the average volume per molecule in a liquid does not depend on the drop size.
2. Nuclear forces. Rutherford's experiments in 1909. by dispersion a-particles have shown that Coulomb's law is fulfilled in the microcosm up to the size of nuclei. But this means that huge repulsive forces must act between the protons in the nucleus. Let us find the magnitude of these forces for two protons in a helium nucleus. The number of nucleons in a helium nucleus. From formula (19.1) the radius of the helium nucleus R\u003d 1.25 10 -15 4 1/3 \u003d 2 10 -15 m. We assume that the protons are at the ends of the nucleus diameter 2 R= 4 10 -15 m. Then the repulsive force between them is:
Why does the nucleus, despite such a large mutual repulsion of protons, not scatter into its component parts?
The experimentally observed stability of nuclei means that in addition to electrical forces repulsion between nuclear particles, there are also forces of attraction. It can't be strength gravitational attraction. Them potential energy
– Gmp/2R= – 6.7 10 -11 (1.7 10 -27) 2 /4 10 -15 = – 5 10 -50 J, while the potential energy of proton repulsion is (1/4 πε
0)/(e 2 /2R) ≈ 6 10 -14 J! This is 36 orders of magnitude higher.
Consequently, in the case of atomic nuclei, we are faced with a new, special kind of interaction. This interaction is called strong, and the forces responding to it - nuclear. Complete theory nuclear forces has not yet been built, although it has achieved great success in explaining and predicting many experimental facts. The main modern ideas about nuclear forces are as follows:
a. Existence of a nuclear field . Just as electrical forces are conditioned by the existence of material electromagnetic field, nuclear forces are due to the existence of a material nuclear field. The sources of the EM field are any electrically charged particles of matter - electrons, protons, etc. The sources of the nuclear field are nucleons - protons and neutrons. Nucleons have a specific nuclear charge.
b. Charge independence of nuclear forces . nuclear charge nucleons is the same in magnitude and sign. It does not depend on whether the nucleon has an electric charge (proton) or not (neutron). With the help of nuclear forces, a neutron with a neutron, a neutron with a proton, and two protons interact in the same way.
in. Short-range nuclear forces. Compared to the Coulomb and gravitational forces, which decrease in proportion to the square of the distance between point sources (charges, masses), nuclear forces decrease much faster. They are very large at distances of the order of the diameter of the nucleus, but already at a distance of three diameters they are practically invisible. When nucleons approach each other, attraction is replaced by repulsion.
G. Nuclear forces are not central. electric field around point charge and the gravitational field around point masses are centrally symmetrical. The nuclear field of an individual nucleon does not have central symmetry. This is due to the presence of spin mechanical and magnetic moments in nucleons. The interaction between nucleons depends on the orientation of their spins. For example, a neutron and a proton are held together in the nucleus of heavy hydrogen - the deuteron - only if their mechanical spins are parallel to each other.
d. Saturation property of nuclear forces. As is known, the interaction energy of two electric charges does not depend on the presence of a third ( superposition principle). With the introduction of each subsequent charge, the energy of the system increases in proportion to the amount of the introduced charge. Each electric charge can interact with an unlimited number of other charges. There is no saturation of electric forces.
The saturation of nuclear forces is that each nucleon in the nucleus can interact with a limited number of nucleons. This is somewhat similar to the chemical valency of the elements. For example, a carbon atom C can combine with four hydrogen atoms H to form a methane molecule CH 4 , with two oxygen atoms (CO 2 ), and so on. There is no such diversity among nucleons as among chemical elements. Therefore, the saturation of nuclear forces manifests itself in the fact that specific binding energy(the energy per nucleon) in the nucleus does not increase with an increase in the number of nucleons in the nucleus, but remains approximately constant.
3. Nucleus mass differs from the mass of an atom of chemical elements only by the value of the mass of the electron shell. In the periodic table, the masses of atoms are expressed in atomic mass units a.m.u. One a.m.u. equals 1 ç 12 masses of a carbon atom, 1 amu = 1.66 10 -27 kg. Since there are 12 nucleons in the core of the main carbon isotope (99% on earth), it is clear that the a.m.u. close to the mass of one nucleon.
Mass of one electron me= 5.5 10 -4 amu The mass ratio of the electron shell Zme to the mass of an atom is for hydrogen me/m() = 5.5 10 -4 ç 1.008 = 0.0005, i.e. 0.05%. For other atoms, this ratio is even smaller, since the number of nucleons in the nucleus grows faster than the number of electrons in the atom. in the uranium atom Zme/m() = 0.0002 (0.02%). Therefore, in cases where calculations require an accuracy of no more than four digits, the mass of an atom of a chemical element from the periodic table can be taken as the mass of the nucleus.
As the nuclear physics previously unknown isotopes, including those that did not exist in nature, were discovered and synthesized through nuclear reactions.
Chemical measurement methods atomic masses artificial isotopes proved little effective. In 1919 Francis Aston constructs mass spectrograph- a device capable of separating ions according to their masses with high accuracy.
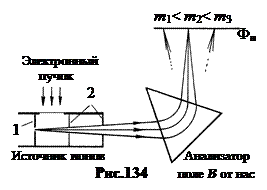 Any mass spectrograph includes three main parts: ion source, analyzer and receiver. Ions are formed in the ion source and a weakly diverging beam is formed. In the analyzer, the beam is divided into several beams, which differ in ion masses and are focused on the surface of the receiving device - a photographic plate. Figure 134 shows a diagram of one of these mass spectrographs. The steam jet of the element under study enters the hole 1 of the ion source and is ionized by the electron beam that shoots through it. The resulting ions are accelerated and collimated by diaphragms 2.
Any mass spectrograph includes three main parts: ion source, analyzer and receiver. Ions are formed in the ion source and a weakly diverging beam is formed. In the analyzer, the beam is divided into several beams, which differ in ion masses and are focused on the surface of the receiving device - a photographic plate. Figure 134 shows a diagram of one of these mass spectrographs. The steam jet of the element under study enters the hole 1 of the ion source and is ionized by the electron beam that shoots through it. The resulting ions are accelerated and collimated by diaphragms 2.
The analyzer is a sector magnetic field AT directed perpendicular to the plane of the figure. In this magnetic field, ions emitted from the source at slightly different angles are deflected and focused. The radius of the circle along which the ion moves in the sector is the larger, the smaller its specific charge, ![]() . (19.2)
. (19.2)
(See Electricity, §14). Here v is the speed of the ion, e/m is its specific charge.
As a result, ions of the same isotope fall into the same place on the photographic plate Фп, forming a narrow line on it, perpendicular to the plane of the picture. Mass spectrographs make it possible to measure the masses of isotopes with a relative error of 10 -5 ¸10 -6 .
If we replace the photographic plate with a Faraday cup and measure the ion current, we can determine the intensity of the ion beams and find the relative abundance of isotopes in the ion mixture. Such a device is called mass spectrometer.
4 . Mass defect and nuclear binding energy. Core mass m i is always less than the masses of its constituent nucleons Zm p+ (A-Z)m n.D value m = Zm p + (A-Z)m n –m i (19.3)
 called mass defect. Here m p,m n,m and i are the rest masses of the proton, neutron and nucleus.
called mass defect. Here m p,m n,m and i are the rest masses of the proton, neutron and nucleus.
The fact is that when free nucleons combine, energy is released in the form of EM radiation quanta, which carry away the mass Δ m. Bond energy E the number of nucleons in a nucleus is calculated by the formula E sv = D mc 2 , (19.4)
where c is the speed of light in vacuum. To destroy the nucleus, that is, to divide it into nucleons, it is necessary to impart energy E, not less than the binding energy, E≥ E St.
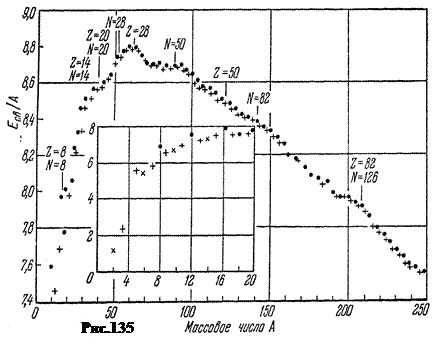
Indicative for assessing the stability of nuclei specific binding energy E St. cA, that is, the energy per 1 nucleon. Figure 135 shows the experimental dependence of its modulus on the mass number A stable nuclei in MeV/nucleon. Black dots refer to even-even nuclei, in which the number of protons Z and the number of neutrons A-Z- even numbers. Straight crosses - to nuclei with an odd A. Oblique crosses refer to odd-odd nuclei.
The inset shows the specific binding energy for light nuclei, starting with deuterium ( A= 2) and ending with neon ( A = 20).
In the first approximation, the specific binding energy varies from 7.4 MeV ç nucleon in deuterium up to 8.8 MeV ç nucleon at iron, that is, within about 1.4 MeV ê nucleon. But the nature of the change is indicative. From deuterium to iron, magnitude E St. cA grows, reaching a maximum at A= 56, that is, in the region of iron nuclei. After iron with growth A specific binding energy drops to 7.5 MeV ç nucleon at the end of the periodic table.
The maximum of the curve corresponds to the most stable nuclei. These include nuclei E St. cA> 8.6 MeV ç nucleon starting with even-even calcium and ending with even-even lead with E St. cA= 7.9 MeV ç nucleon. The lightest nuclei with A < 20 энергетически выгодно сливаться друг с другом в более тяжёлые с выделением fusion energy. For the heaviest nuclei with A> 207, on the contrary, the process of fission into fragments is beneficial, which proceeds with the release of energy, which is called atomic.
Interestingly, the nuclei of some elements in Fig. 135 are slightly higher than the course of the curve. These are nuclei in which the number of protons Z or the number of neutrons N=A-Z equal to the so-called magic numbers: 2, 8, 20, 28, 50, 82, 126. These cores have increased strength compared to their neighbors. Doubly magic nuclei are especially strong. Of these, the nucleus is so strong that even during the decay of heavy nuclei it flies out intact ( a-particle).
5 . Spins of nuclei. As already mentioned in §18, protons and neutrons have spin mechanical moments, the same as those of electrons. In the projection onto the physical axis, the spin mechanical moments of the proton, neutron, and electron can take on the values L sz = ± ћç 2.
All methods for experimental determination of spin moments Ls protons, neutrons and nuclei are based on the connection of mechanical spin moments Ls with magnetic spin moments Ms. Knowing the relationship between Ls and Ms for these particles, according to the magnitude and features of the magnetic moment M s, it is possible to establish the mechanical Ls.
Earliest experimental methods determination of magnetic spin moments M s were based on the study of the hyperfine structure of the optical spectra of hydrogen (proton spin), deuterium (nucleus spin proton + neutron → neutron spin) and other atoms. Later, they began to study the behavior of nuclei in a magnetic field by radiospectroscopy. The spins of the nuclei of short-lived isotopes are determined by nuclear reactions on the basis of conservation laws, the spins of excited nuclei - according to the emitted by them g-radiation.
Experiments show that the spin moments of the nucleus (mechanical and magnetic, respectively) are equal to the geometric sums of the moments of the nucleons that make up the nucleus. It should be borne in mind that the total momentum of each nucleon is the sum of the spin and orbital (that is, associated with the movement of the nucleon along a certain “orbit” in the nucleus) moments. The orbital mechanical moment of a nucleon in a nucleus, in contrast to the spin moment, can take only integer values. If a L sz= ± ћç 2, then L lz =± nћ, where n is an integer. Let us list the main experimental facts on nuclear spins.
a. Spin mechanical moments of nuclei with even A is always integer, with odd A - half-integer . For example, the spin of the deuteron L sz = ± ( ћç 2+ћç 2) = ± ћ . The spins of a neutron and a proton in a deuterium nucleus are parallel.
b. The spin mechanical moments of all even-even nuclei in the ground states are equal to zero . Since the spins in the proton - neutron pair of deuterium are parallel, it remains to conclude that antiparallel spins can only be in pairs of the same name, that is, for proton-proton and neutron-neutron.
in. The spin mechanical moments of the remaining stable nuclei do not exceed 9ћç 2, that is, they are very small compared to the sum of the absolute values of the spin and orbital moments of all particles entering the nucleus. This suggests that most of the nucleons are strongly bound in closed shells that have zero total momentum and do not participate in the creation of the nuclear spin.
6. Magnetic moments of nuclei. Every nucleus with non-zero spin has a magnetic moment M characterizing the interaction of the nucleus with a uniform external magnetic field B . Direction of magnetic moment M microparticles coincides with the direction of the spin mechanical moment Ls up to sign. M s = g Ls (19.5)
Here g – gyromagnetic ratio. It is positive for the proton (vectors Ms and Ls coincide, for the neutron it is negative (vectors Ms and Ls are opposite). There are many methods for determining the magnetic moments of nuclei and nucleons. Let's consider three of them.
a. Study of the hyperfine structure of optical spectra. This method has already been mentioned in the previous paragraph. We only add that in this way it was possible to establish that the nuclear magnetic moments are three orders of magnitude smaller than the spin magnetic moment of the electron and have the order nuclear magneton M i = eћç 2m p= 5.05 10 -27 J ç Tl. That's why hyperfine splitting spectral lines three orders of magnitude less fine structure, due to the interaction of the spin moment of the electron with the orbital one.
 b. Nuclear magnetic resonance method
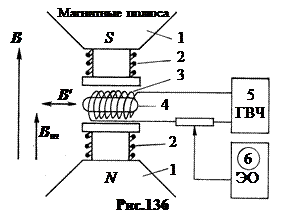
lies in the fact that the spin of a nucleus located in a strong constant magnetic field can “overturn” under the action of a weak high-frequency field of a certain resonant frequency. The installation diagram is shown in Fig.136.
b. Nuclear magnetic resonance method
lies in the fact that the spin of a nucleus located in a strong constant magnetic field can “overturn” under the action of a weak high-frequency field of a certain resonant frequency. The installation diagram is shown in Fig.136.
 Three magnetic fields. First, a strong permanent magnetic field B≈ 1 T created by magnets 1. Purpose of the magnetic field B
is the breaking of the connection between the nuclear magnetic moment and electron shell, creation of hyperfine Zeeman splitting of nuclear levels. Secondly, the high-frequency field created by the coil 3 from the generator 5 B
"
, directed perpendicular B
. Field frequency B
"
may change. The purpose of this field is the resonant breaking of the nuclear spin. Thirdly, the modulating variable field created by the coils 2 B
m, directed parallel B
and having a low constant frequency of 50 Hz. The purpose of this field is to repeat the reversal of the nuclear spin 50 times per second in order to obtain a stationary picture on the oscilloscope 6 at resonance. By measuring the energy absorbed at resonance by the sample, the induction of a strong magnetic field B
and the frequency of the generator, it is possible to determine the magnetic moment of the nuclei on modern installations with an accuracy of 6 decimal places.
Three magnetic fields. First, a strong permanent magnetic field B≈ 1 T created by magnets 1. Purpose of the magnetic field B
is the breaking of the connection between the nuclear magnetic moment and electron shell, creation of hyperfine Zeeman splitting of nuclear levels. Secondly, the high-frequency field created by the coil 3 from the generator 5 B
"
, directed perpendicular B
. Field frequency B
"
may change. The purpose of this field is the resonant breaking of the nuclear spin. Thirdly, the modulating variable field created by the coils 2 B
m, directed parallel B
and having a low constant frequency of 50 Hz. The purpose of this field is to repeat the reversal of the nuclear spin 50 times per second in order to obtain a stationary picture on the oscilloscope 6 at resonance. By measuring the energy absorbed at resonance by the sample, the induction of a strong magnetic field B
and the frequency of the generator, it is possible to determine the magnetic moment of the nuclei on modern installations with an accuracy of 6 decimal places.
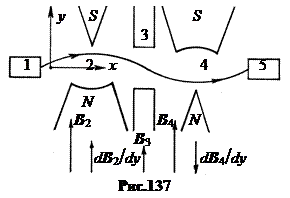
in. Isidor Rabi method, 1937 The method of nuclear magnetic resonance cannot measure the magnetic moment of a neutron, since neutrons are not kept in an ampoule, they exist only in beams. The scheme of the Rabi method is shown in Fig. 137. The neutron beam from source 1 successively passes through two highly inhomogeneous magnetic fields 2 and 4 with oppositely directed gradients dBcdy. In an inhomogeneous magnetic field, the trajectory of a particle with a spin magnetic moment is curved along or against the gradient, depending on the orientation of the magnetic moment. The first magnetic field 2 bends the trajectory of the neutrons, the second field 4 focuses them on the detector 5. If now, in the gap 3 between the fields, we include a combination of constant strong homogeneous and weak high-frequency magnetic fields from the previous scheme, then the neutron spin will flip at resonance. As a result, these neutrons will not be focused by the second inhomogeneous field 4 and will not fall into the detector 5. Therefore, there will be a sharp dip in the graph of the counting intensity in the detector depending on the frequency of the high-frequency field at resonance. Its position will determine the magnitude of the magnetic moment of the neutron.
The Rabi method can also use molecular beams and determine the magnetic moments of nuclei.
Let us list the main experimental facts on the magnetic moments of nucleons and atomic nuclei.
a. Magnetic spin moments of the proton Mp and neutron M n are: Mp= 2,79 M i, M n = –1,91 M i. Here M i = ehç 2m p= 5.05 10 -27 J ç Tl is the nuclear magneton.
b . The magnetic moments of nuclei with zero spin are zero.
in . The magnetic moments of nuclei with nonzero spin are of the order of the nuclear magneton.
G. Spin magnetic moments of nucleons are not additive. For example, the deuteron consists of a proton and a neutron with parallel spins. The total magnetic moment of the deuteron should be M d = M p + M n= 2,79 M i – 1,91 M i = 0,88 M i. But experience gives M d = 0,86 M i. This non-additivity is connected with the non-centrality of the forces acting between nucleons.
7 . Models of the atomic nucleus. A consistent theory of the nucleus has not yet been built due to two main difficulties: insufficient knowledge of the forces acting between nucleons, and the cumbersomeness quantum problem many bodies. After all, a nucleus with a mass number A must be described by a system consisting of at least A equations. These difficulties can be partially overcome by creating nuclear models that make it possible to describe with the help of simple equations a certain set of properties of the nucleus.
About a dozen models have been developed, each of which describes its own set of nuclear properties and its own range of phenomena. Let's consider two of them.
a. drip model. She was offered Yakov Frenkel in 1937. The nucleus in this model is likened to a drop of liquid, the excited nucleus is likened to a heated drop. If E is the energy of an excited nucleus with the number of nucleons A, then, having assigned 3 degrees of freedom to nucleons, we obtain:
![]() . (19.6)
. (19.6)
At E = 10 MeV T≈ 10 9 K. Emission of neutrons, protons and a-particles in such a model can be interpreted as the evaporation of the drop core.
The basis for the drop model was the short-range action of nuclear forces and the independence of density from the mass number A. The drop model made it possible to derive a semi-empirical formula for the binding energy of particles in a nucleus and describe the process of fission of heavy nuclei.
b. Shell model. She was proposed in 1951. Maria Goeppert-Mayer. The basis for the shell model was the fact of the existence of especially stable nuclei, the so-called magical and doubly magical.
In this model, nucleons are considered to move independently of each other in averaged centrally symmetric field. It is assumed that, as in the atom, the nucleus has discrete levels, filled with nucleons, taking into account the Pauli principle. These levels are grouped into shells, each of which can contain a certain number of nucleons. A completely filled shell forms a stable core.
From the experimental fact of the existence of magic and doubly magic nuclei, it follows that the filled shells contain the magic number of nucleons 2, 8, 20, 28, 50, 82, 126. Many magic nuclei are more common in the Universe than their non-magic neighbors. In addition to twice magical (with the exception of), this includes
The shell model is well developed mathematically and allows one to explain the properties of magic nuclei and neighboring nuclei that differ by 1 nucleon (missing or excess). In this model, the real forces acting between nucleons are replaced by a self-consistent field in which nucleons move independently of each other. The scheme of building shells is somewhat reminiscent of filling in the periodic table, but inferior to it in severity.
Rutherford's experiments proved that the atom consists of a small positively charged nucleus and electrons revolving around it. It turned out that compared to the size of the atom itself, the nucleus is extremely small. The nucleus is 100,000 times smaller than an atom.
If the atomic nucleus is increased to the size of a pea, then the diameter of the atom will be equal to the height of the Ostankino tower.
Further studies have shown that the charge of the atomic nucleus is equal to the product of the serial number Z of the element in the periodic table of D.I. Mendeleev on elementary charge. That is atomic number Z determines the number of electrons in an atom, the number of protons in the nucleus.

If the nucleus consisted of only protons, then the mass of the nucleus of any chemical element would be equal to the mass Z of protons. But in fact, the mass of the nuclei of all elements is much larger. Therefore, in 1920, Rutherford suggested the existence of an electrically neutral particle. This particle was later discovered experimentally. They called her neutron.
In 1932 they proposed proton-neutron model atomic nucleus. Protons and neutrons are called nucleons.
The total number of nucleons (protons + neutrons) is called mass number A. At present, the following designations are accepted for atoms of chemical elements:
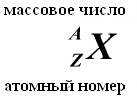
Core Stability
Why is the core stable? Coulomb repulsive forces of enormous magnitude, about 230 N, act between protons inside the nucleus. But the nucleus does not fall apart! The reason for stability is the presence of forces of a different nature, they are called strong interactions.
These forces are 100 times greater than the Coulomb repulsive forces. They appear only at distances of the order of 10 -15 m. They are only forces of attraction.
Core mass
The masses of all nuclei (except hydrogen) are less than the masses of the free protons and neutrons that form them. The mass difference is called mass defect.
The discovery of the neutron immediately led to a change in the idea of the structure of the atomic nucleus. The hypothesis that the nucleus contains protons and electrons, which existed before the discovery of the neutron, was not supported by most scientists. In 1932, surprisingly prolific for outstanding discoveries, the Russian scientist Dmitry Dmitrievich Ivanenko put forward a hypothesis about the proton-neutron model of the nucleus, the electrons in his model were not part of the nucleus. Somewhat later, Heisenberg expressed a similar model of the nucleus. It must be said that this model was accepted skeptically by many scientists. It seemed to them that it contradicted the emission of electrons during P-decay. Heisenberg recalled that "he was heavily criticized by the most eminent physicists." Ivanenko spent a lot of effort both on convincing scientists of the correctness of his discovery and on defending his priority. Soon the proton-neutron model of the nucleus was recognized and became generally accepted, although the nucleus of the atom still contains many secrets. Let's say a few words about the particles that make up the nucleus of an atom. (It should be remembered that both protons and neutrons, according to quantum mechanics, also have wave properties).
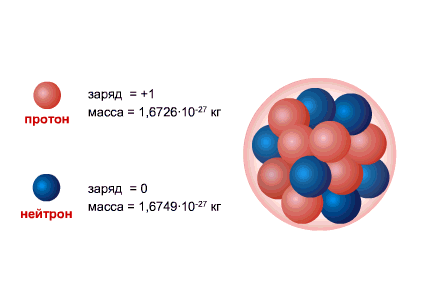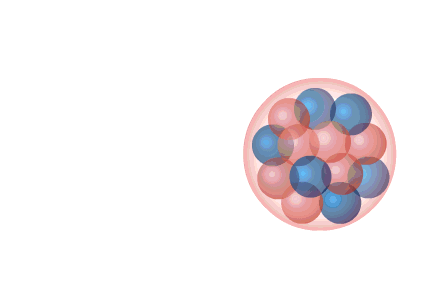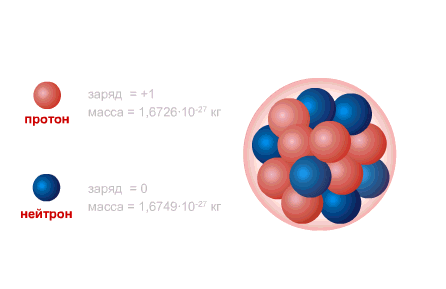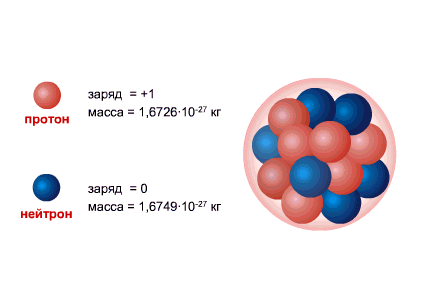
PROTON is the nucleus of the hydrogen atom, its charge is equal to the electron charge in magnitude and opposite in sign (+e = 1.6 x 10-19 C.), and the mass (mp = 1.6726485 ± 0.0000086) x 10- 27 kg.
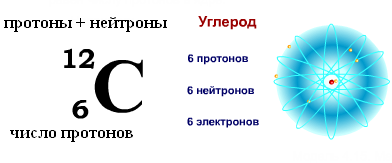
The NEUTRON has a charge equal to zero and a mass practically equal to the mass of a proton (mm = 1.674954 ± 0.000009) x 10-27 kg. 1, Neutrons and protons received common name nucleons. The total number of nucleons in the nucleus, i.e. the number of protons and neutrons in the nucleus, is called the mass number and is denoted by the letter A. The number of protons in the nucleus is denoted by Z. It is called the atomic number and is also equal to the number of electrons in the outer shell of the atom. The number of neutrons in the nucleus N = A - Z.
Chemical elements, at the suggestion of the Joliot-Curie spouses, are designated with two indices to the left of the element designation: The upper index denotes the mass number, the lower index denotes the number of protons, for example, 13 Al, 11 H. In the nuclei of the same chemical element, the number of neutrons can be different, and the number of protons is the same. Such nuclei, which have the same number of protons but a different number of neutrons, are called isotopes. Some isotopes do not occur in nature, but can be obtained in scientists' laboratories as a result of nuclear reactions.
Atomic nucleus- central part atom, in which its main weight(over 99.9%). The nucleus is positively charged, the charge of the nucleus determines chemical element to which the atom belongs. The sizes of the nuclei of various atoms are several femtometers, which is more than 10 thousand times smaller than the size of the atom itself.
Atomic nuclei studies nuclear physics.
The atomic nucleus is made up of nucleons- positively charged protons and neutral neutrons, which are interconnected by means of strong interaction. The proton and neutron have their own angular momentum ( back), equal to [sn 1] and related magnetic moment. The only atom that does not contain a neutron in the nucleus is light hydrogen ( protium).
The atomic nucleus, considered as a class of particles with a certain number of protons and neutrons, is commonly called nuclide .
The number of protons in a nucleus is called its charge number- this number is equal to the serial number element to which the atom belongs table (Periodic system of elements) Mendeleev. The number of protons in the nucleus determines the structure electron shell neutral atom and thus Chemical properties the corresponding element. The number of neutrons in a nucleus is called its isotopic number. Nuclei with the same number of protons and different numbers of neutrons are called isotopes. Nuclei with the same number of neutrons but different numbers of protons are called isotones. The terms isotope and isotone are also used in relation to atoms containing the indicated nuclei, as well as to characterize non-chemical varieties of one chemical element. The total number of nucleons in a nucleus is called its mass number() and is approximately equal to the average mass of an atom, indicated in the periodic table. Nuclides with the same mass number but different proton-neutron composition are called isobars.
Like any quantum system, nuclei can be in a metastable excited state, and in some cases lifetime this state is calculated in years. Such excited states of nuclei are called nuclear isomers .
Rutherford's experiments proved that the atom consists of a small positively charged nucleus and electrons revolving around it. It turned out that compared to the size of the atom itself, the nucleus is extremely small. The nucleus is 100,000 times smaller than an atom.
If the atomic nucleus is increased to the size of a pea, then the diameter of the atom will be equal to the height of the Ostankino tower.
Further studies have shown that the charge of the atomic nucleus is equal to the product of the serial number Z of the element in the periodic table of D.I. Mendeleev on the elementary charge. That is atomic number Z determines the number of electrons in an atom, the number of protons in the nucleus.

If the nucleus consisted of only protons, then the mass of the nucleus of any chemical element would be equal to the mass Z of protons. But in fact, the mass of the nuclei of all elements is much larger. Therefore, in 1920, Rutherford suggested the existence of an electrically neutral particle. This particle was later discovered experimentally. They called her neutron.
In 1932 they proposed proton-neutron model atomic nucleus. Protons and neutrons are called nucleons.

The total number of nucleons (protons + neutrons) is called mass number A. At present, the following designations are accepted for atoms of chemical elements:
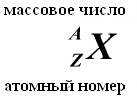

Core Stability
Why is the core stable? Coulomb repulsive forces of enormous magnitude, about 230 N, act between protons inside the nucleus. But the nucleus does not fall apart! The reason for stability is the presence of forces of a different nature, they are called strong interactions.
These forces are 100 times greater than the Coulomb repulsive forces. They appear only at distances of the order of 10 -15 m. They are only forces of attraction.
"The structure of the atomic nucleus" - Ernest Rutherford (structure of the atom). You can die from the invisible. Becquerel Antoine Henri - 1897 "Hero with short arms" Mass defect?m ENERGY!!! Igor Vasilievich Kurchatov. The structure of the atom. biological effect on the body. The structure of the atomic nucleus. Radioactivity - spontaneous radiation (the action of uranium salts on a photographic plate).
"The structure of the nucleus of the atom" - Nuclear reactor. Radioactive transformation of atomic nuclei. Mass numbers chemical elements. The structure of the atom. Uranium nuclei are bombarded with neutrons. Rutherford model of the atom. Foil from the investigated metal. The composition of the atomic nucleus. Rutherford's experiments 1. A grain of radium was placed in a thick-walled lead vessel.
““The structure of the nucleus” of physics” - Proactinius. Proton-neutron model of the atomic nucleus. Half life. Learn about the history of the discovery of the neutron. Charge number. radiation. How many nucleons do nuclei contain. The structure of the atomic nucleus. Unknown product. Device. Isotopes. Particle. Neutron. Radiation. The structure of the atom. Helium core. New element.
"The structure of the atom and the atomic nucleus" - Energy level(electronic layer). Write an electronic formula. Distribution of electrons over sublevels. The composition of the atom. Examples of electronic formulas of atoms. Goals. Electronic graphic diagram of the nitrogen atom. Finding an electron in an atom. Opening the core. Image of electron orbitals. Rules for the distribution of electrons at the energy level.
"The composition of the atomic nucleus" - NUCLEAR FORCES - attractive forces that bind protons and neutrons in the nucleus. The charge number is equal to the charge of the nucleus, expressed in elementary electric charges. The charge number is equal to the ordinal number of the chemical element. Tasks. Does not depend on the presence of a charge. Lesson plan. General form core designations. The composition of the atomic nucleus.
"Composition of the nucleus of the atom" - Dimensions of atomic nuclei. The composition of the nucleus of an atom. Core charge. Proton-neutron model of the nucleus. The nucleus of an atom of a chemical element. Proton and neutron. The nucleus of an atom. Radioactivity. Graph of the specific bond of nucleons in the nucleus. Scheme of Rutherford's experiments. Charge number. Properties of nuclear forces. The number of neutrons in the nucleus of an atom. Discovery of the neutron.
In total there are 7 presentations in the topic






