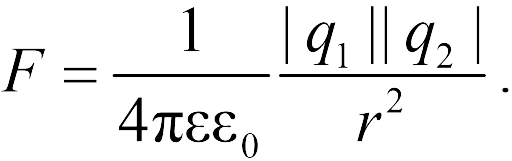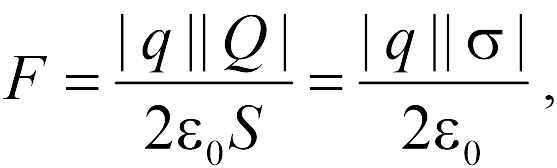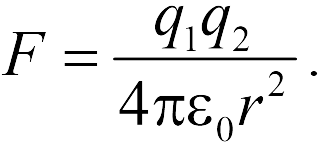The strength of the pendant is. Electric charges. point charge. Coulomb's law
The basic law of interaction of electric charges was found by Charles Coulomb in 1785 experimentally. Coulomb found that the force of interaction between two small charged metal balls is inversely proportional to the square of the distance 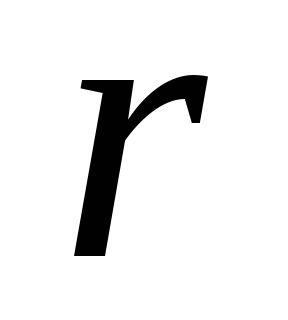 between them and depends on the magnitude of the charges
between them and depends on the magnitude of the charges 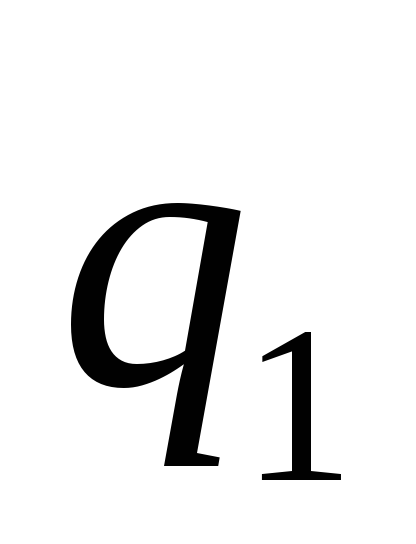 and
and 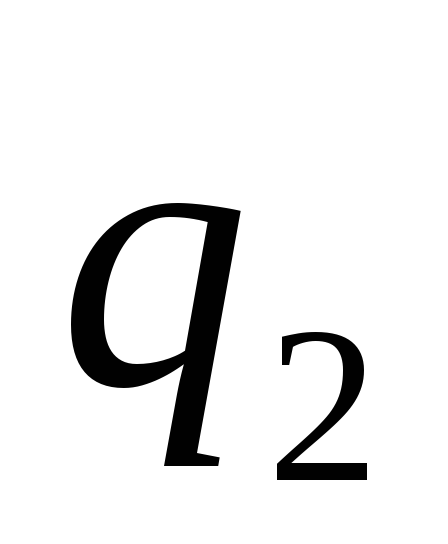 :
:
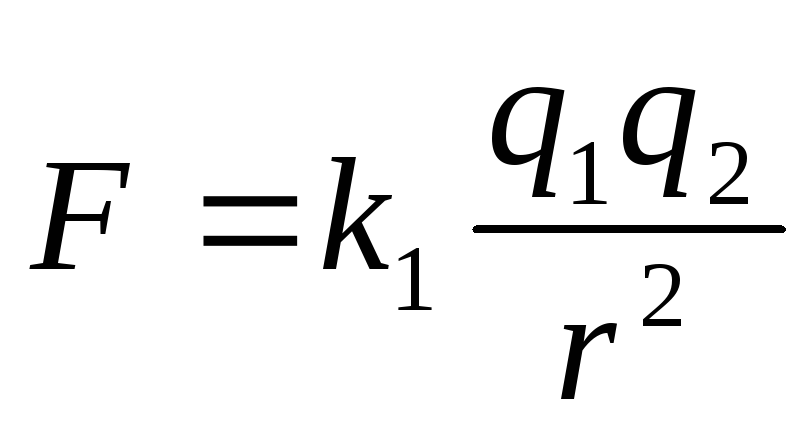 ,
,
where 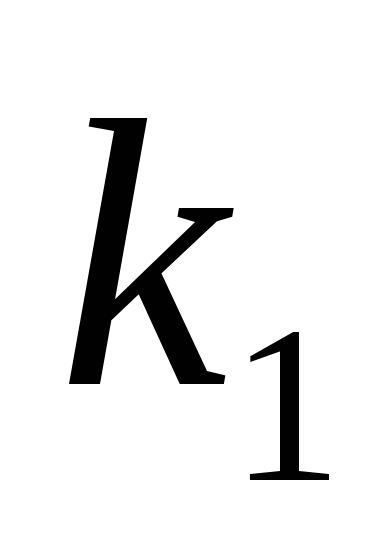 -proportionality factor
-proportionality factor
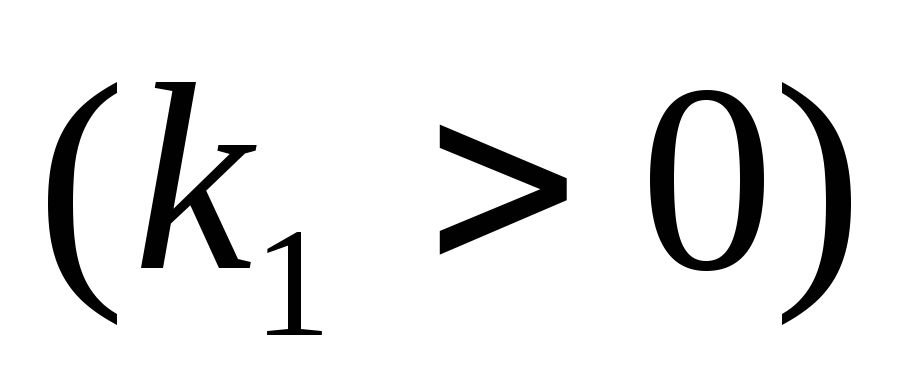 .
.
Forces acting on charges, are central
, that is, they are directed along the straight line connecting the charges. 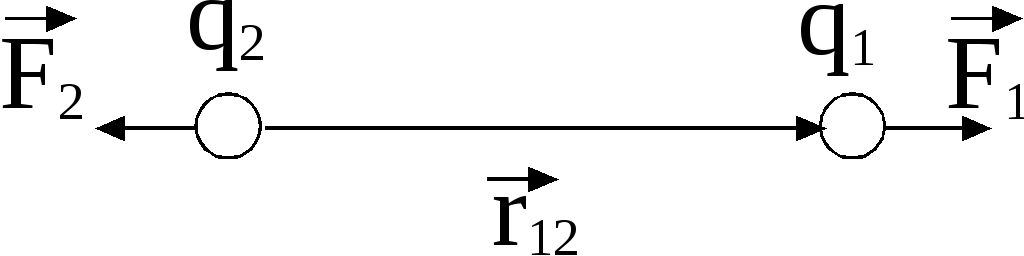
Coulomb's law can be written in vector form: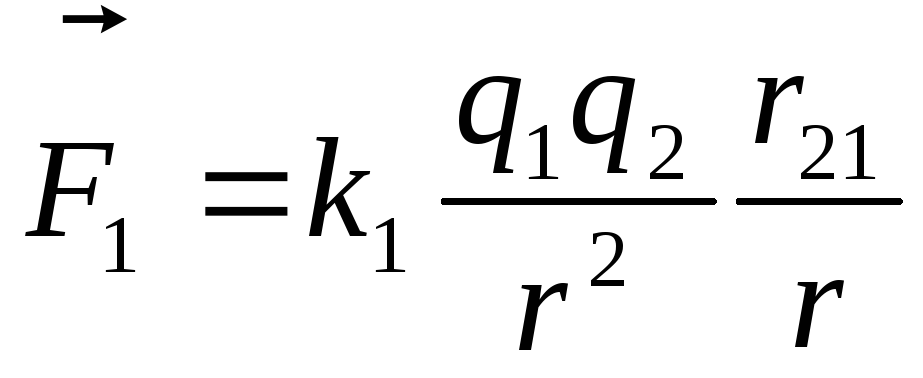 ,
,
where 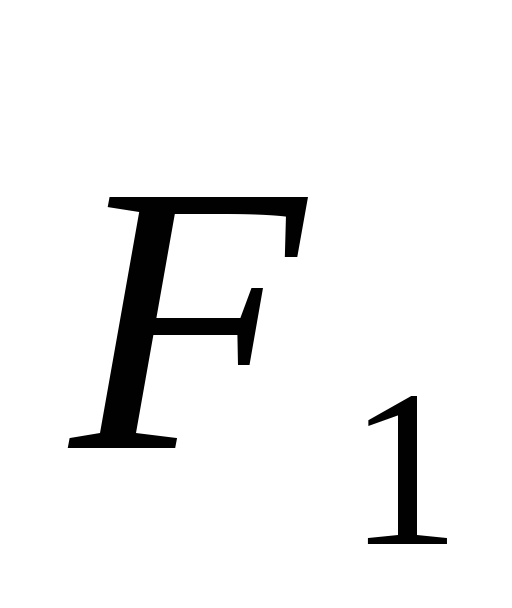 -
-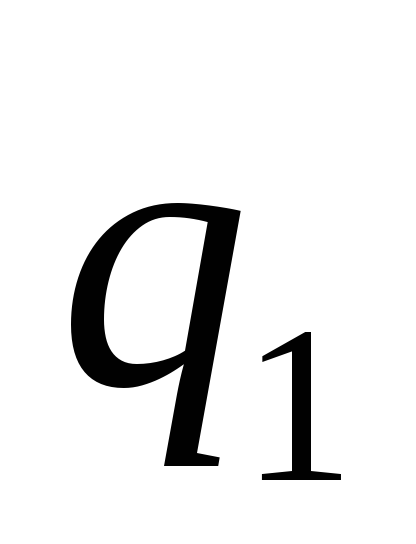 charge side
charge side 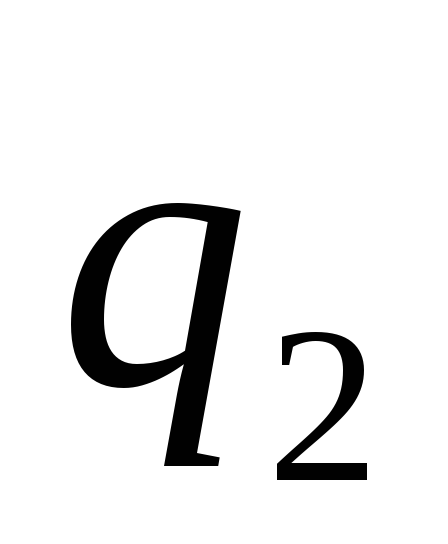 ,
,
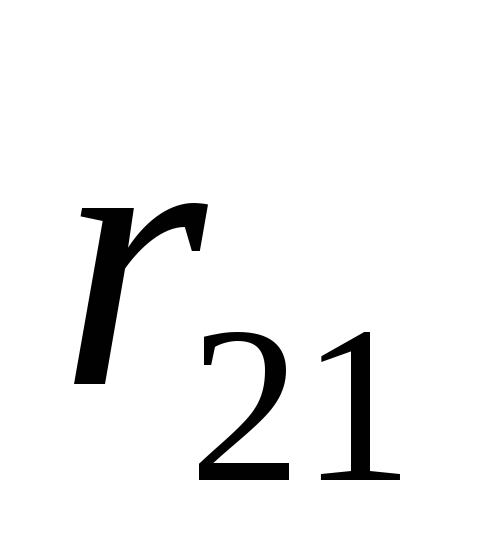 is the radius vector connecting the charge
is the radius vector connecting the charge 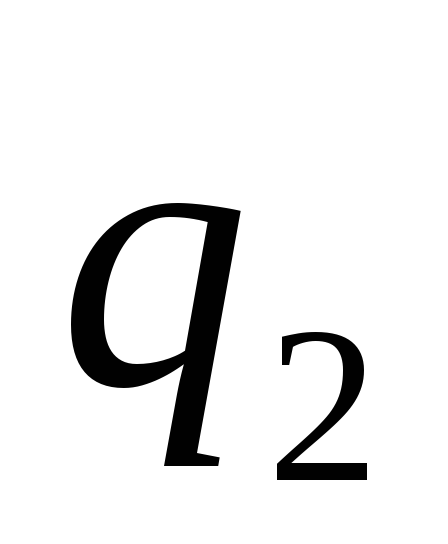 with charge
with charge 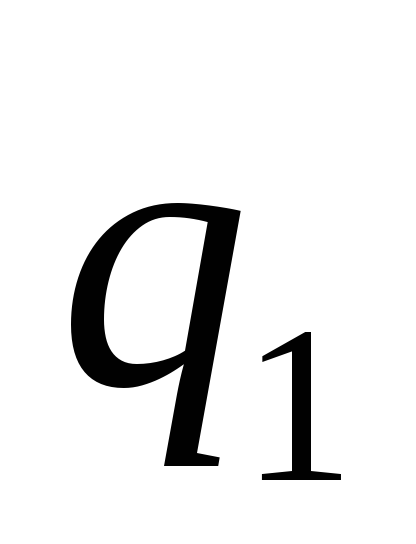 ;
;
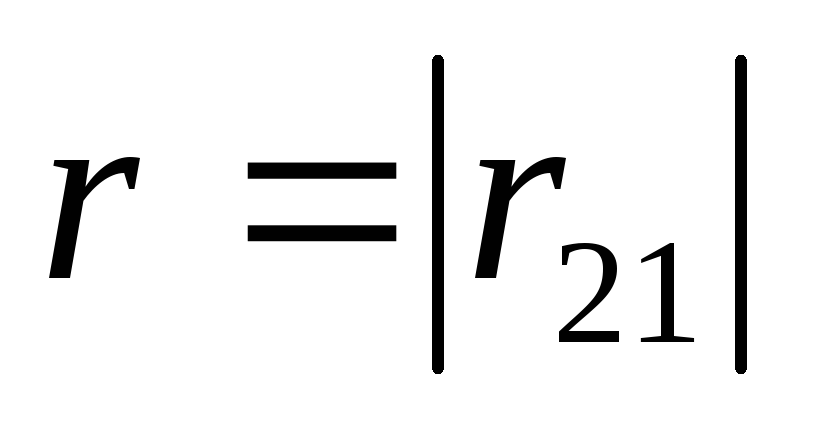 is the modulus of the radius vector.
is the modulus of the radius vector.
Force acting on a charge 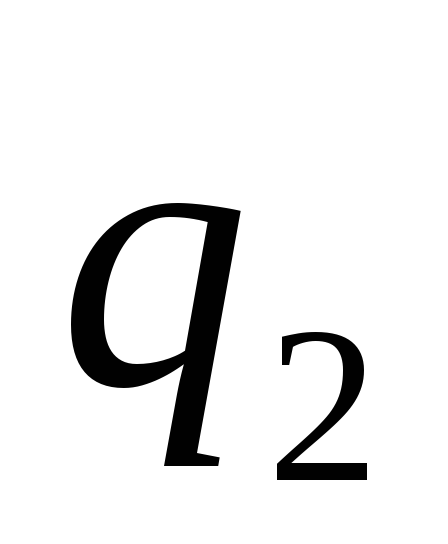 from the side
from the side 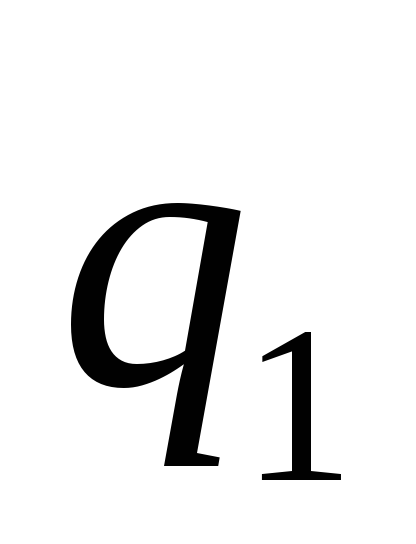 is equal to
is equal to 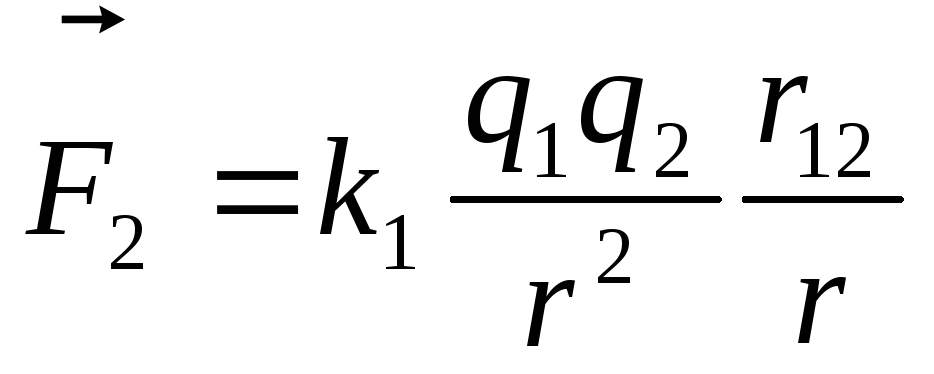 ,
,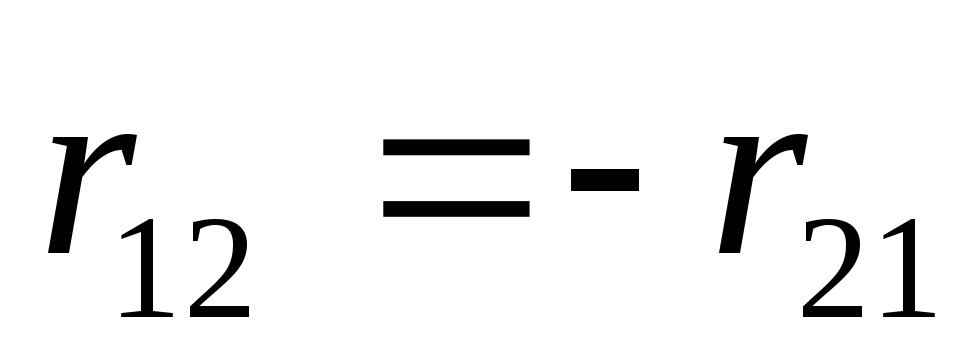 .
.
Coulomb's law in this form
fair only for the interaction of point electric charges, that is, such charged bodies, the linear dimensions of which can be neglected in comparison with the distance between them.
expresses the strength of the interaction between fixed electric charges, that is, this is the electrostatic law.
Formulation of Coulomb's law:
The strength of the electrostatic interaction between two point electric charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between them.
Proportionality factor
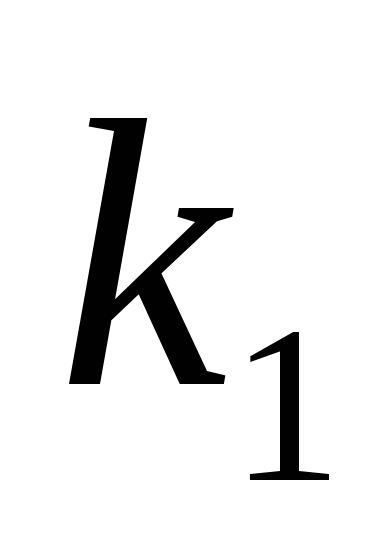 in Coulomb's law depends
in Coulomb's law depends
from the properties of the environment
selection of units of measure for the quantities included in the formula.
That's why 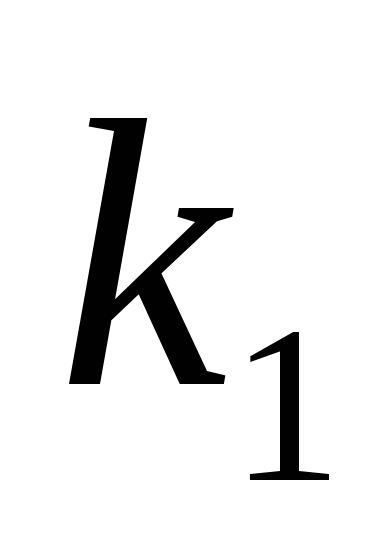 can be represented by the relation
can be represented by the relation 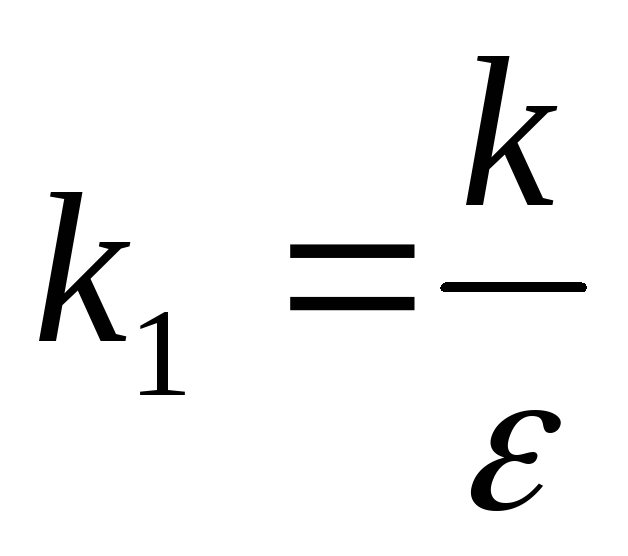 ,
,
where 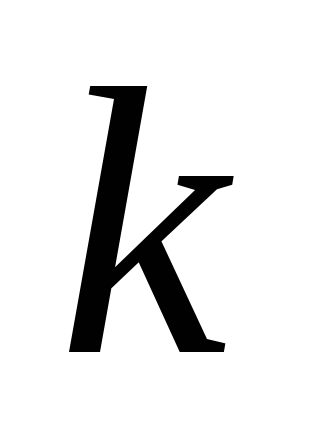 -coefficient depending only on the choice of system of units;
-coefficient depending only on the choice of system of units;
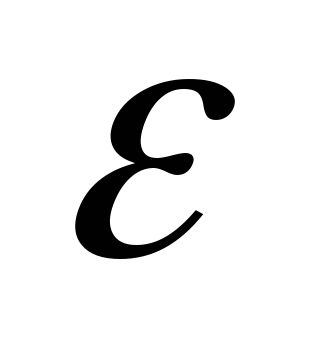 - a dimensionless quantity characterizing the electrical properties of the medium is called relative permittivity environments
. It does not depend on the choice of the system of units and is equal to one in vacuum.
- a dimensionless quantity characterizing the electrical properties of the medium is called relative permittivity environments
. It does not depend on the choice of the system of units and is equal to one in vacuum.
Then Coulomb's law takes the form: 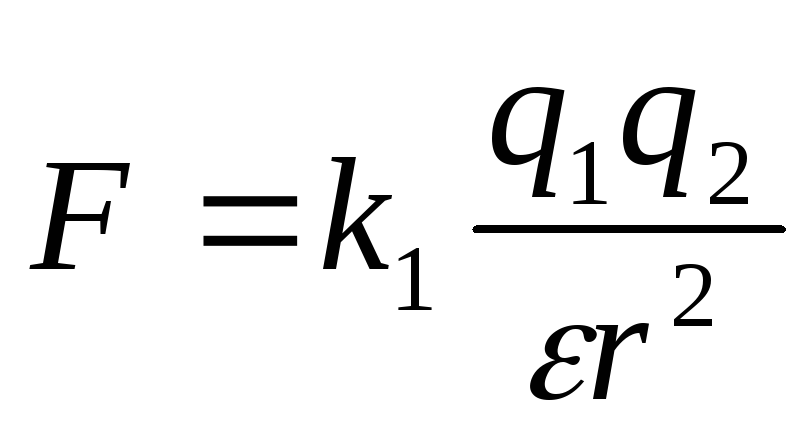 ,
,
for vacuum 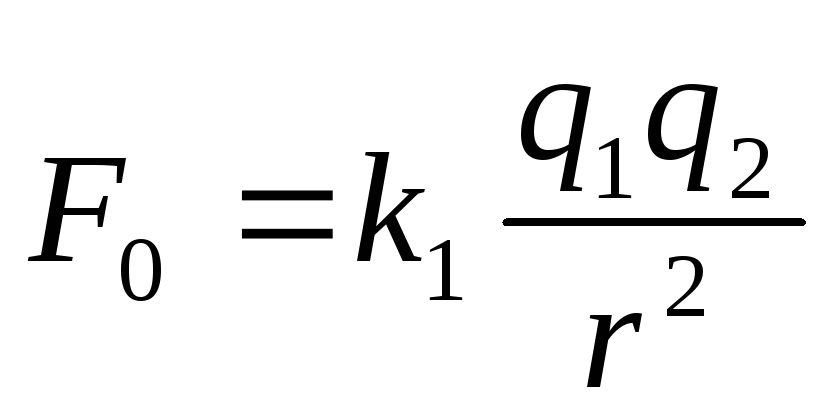 ,
,
then 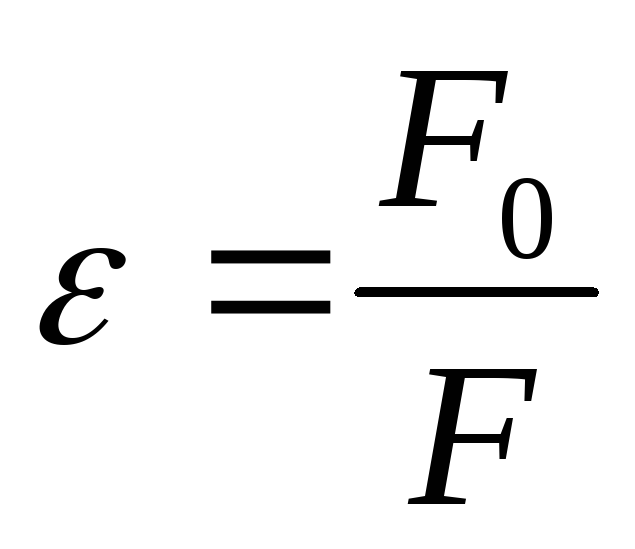 -the relative permittivity of a medium shows how many times in a given medium the force of interaction between two point electric charges
-the relative permittivity of a medium shows how many times in a given medium the force of interaction between two point electric charges 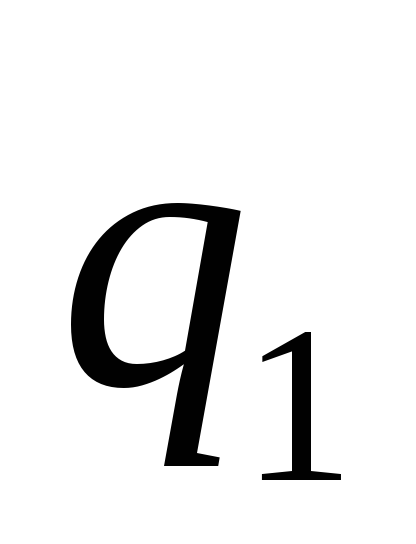 and
and 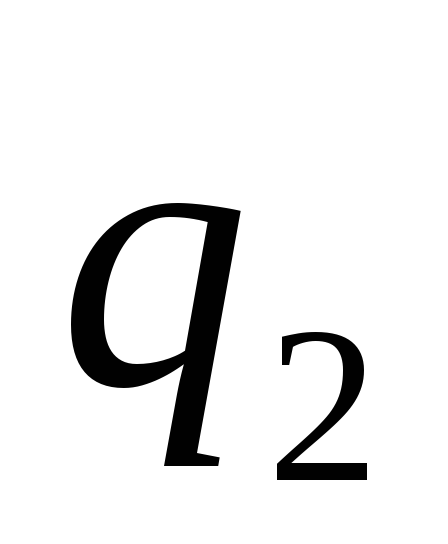 , located at a distance from each other
, located at a distance from each other 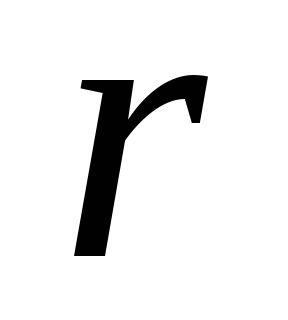 , less than in vacuum.
, less than in vacuum.
In the SI system coefficient 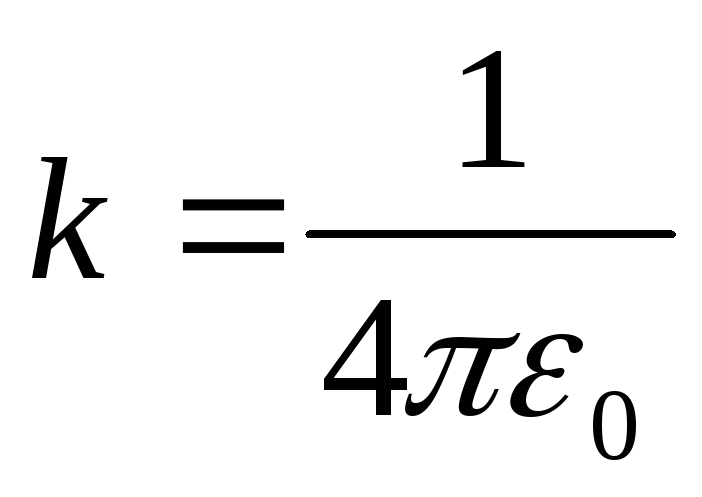 , and
, and
Coulomb's law has the form: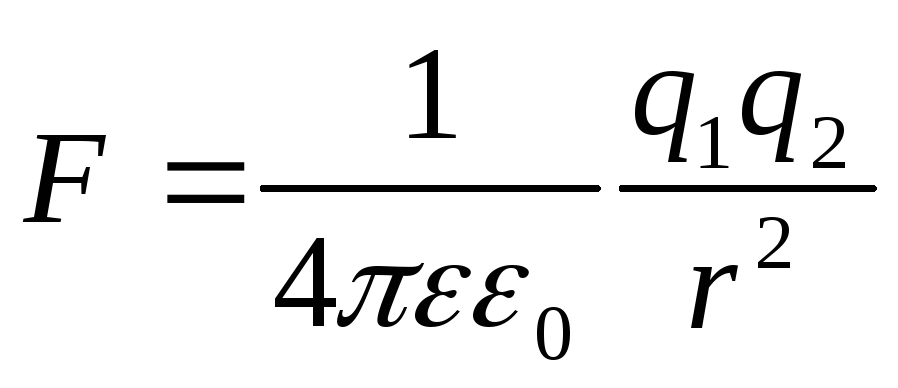 .
.
it rationalized notation of the law K oolon.
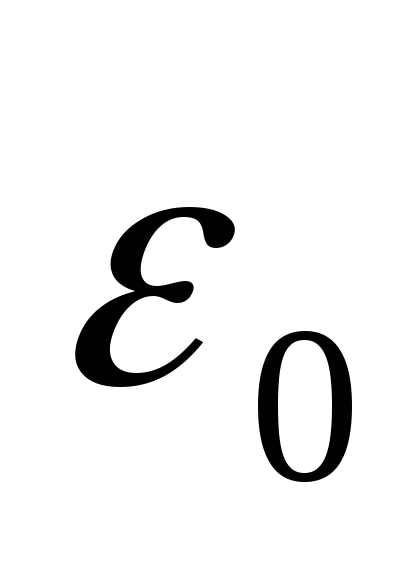 - electrical constant,
- electrical constant, 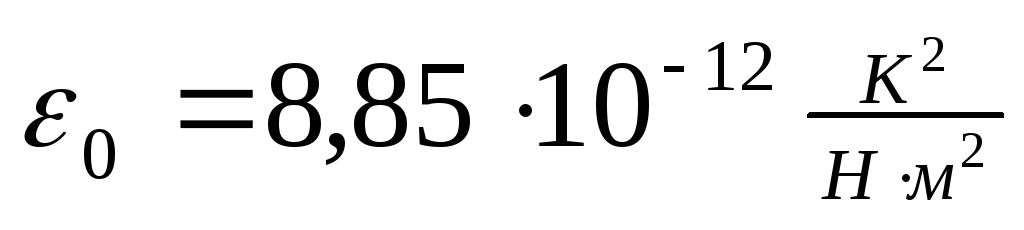 .
.
In the GSSE system
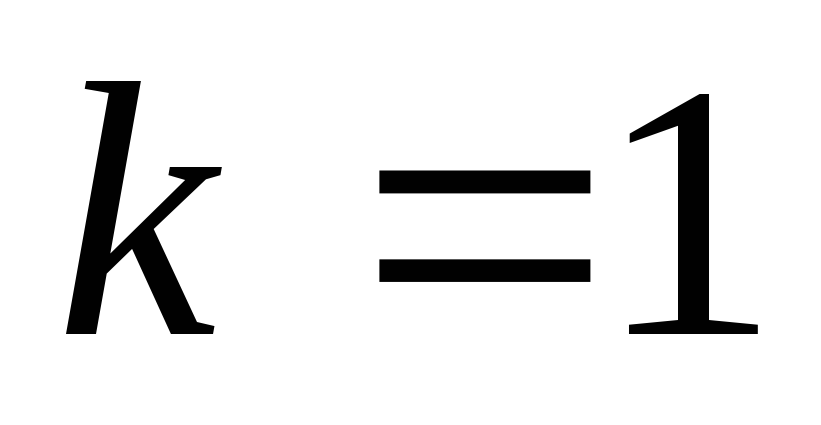 ,
,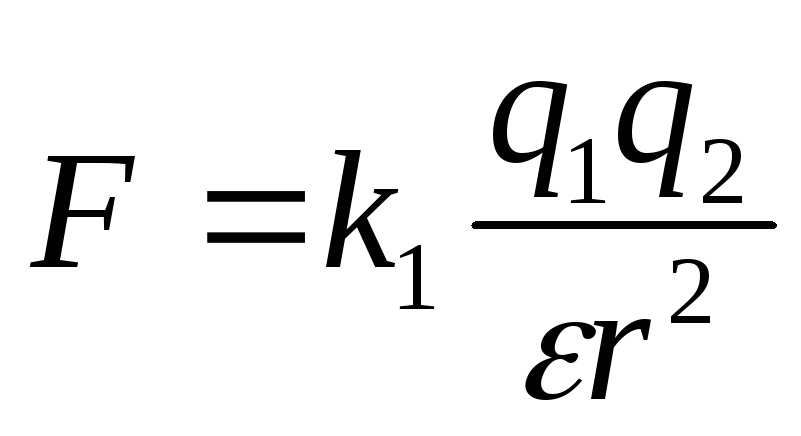 .
.
In vector form, Coulomb's law takes the form 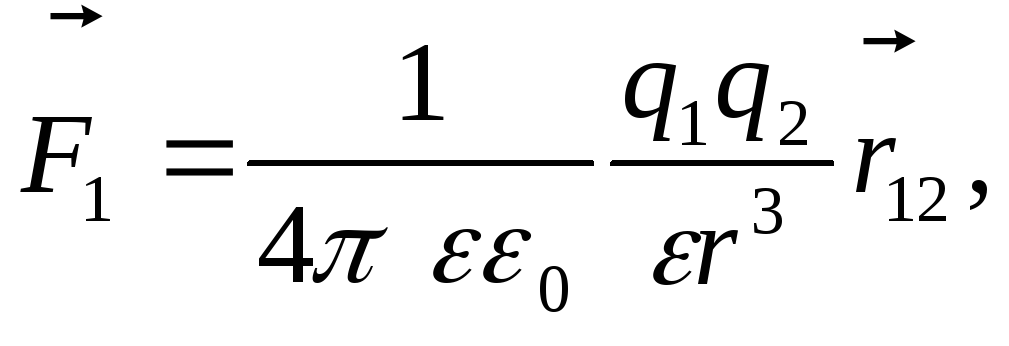
where 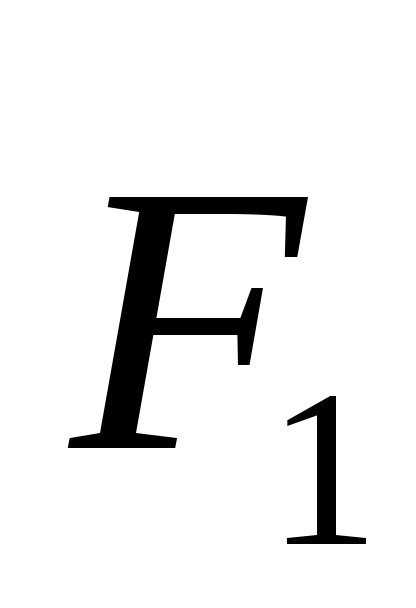 -the vector of the force acting on the charge
-the vector of the force acting on the charge 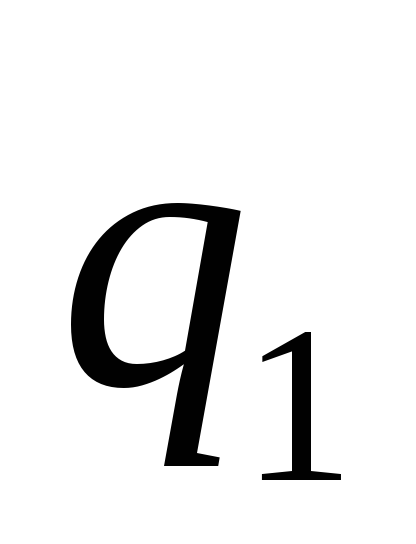 charge side
charge side
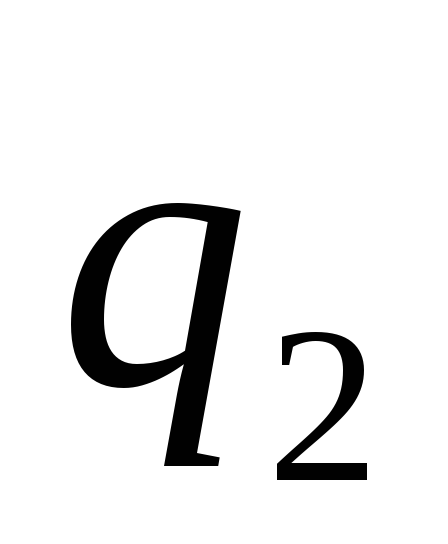 ,
,
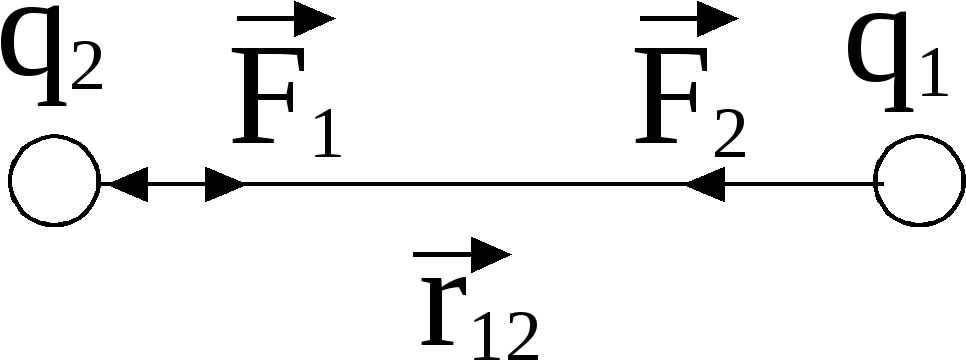 is the radius vector connecting the charge
is the radius vector connecting the charge 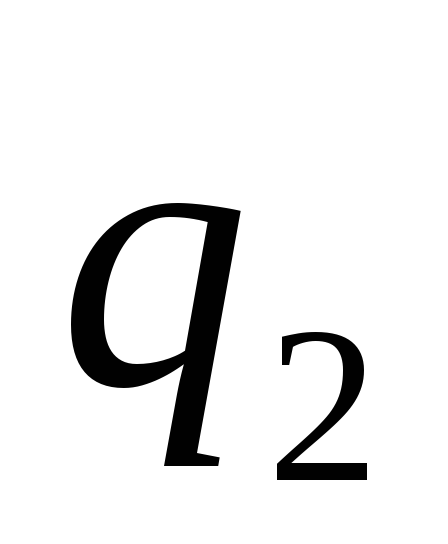 with charge
with charge
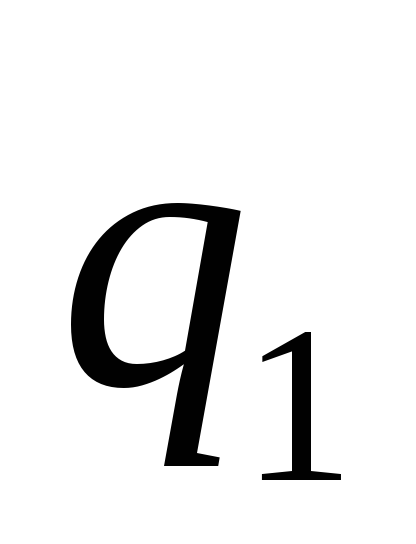
r is the modulus of the radius vector 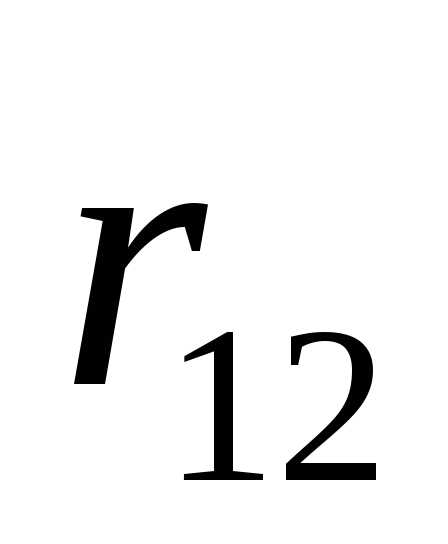 .
.
Any charged body consists of many point electric charges, so the electrostatic force with which one charged body acts on another is equal to the vector sum of the forces applied to all point charges of the second body from each point charge of the first body.
1.3 Electric field. Tension.
Space, in which there is an electric charge, has certain physical properties.
For everyone another the charge introduced into this space is acted upon by electrostatic Coulomb forces.
If a force acts at every point in space, then we say that there is a force field in this space.
The field, along with matter, is a form of matter.
If the field is stationary, that is, does not change in time, and is created by stationary electric charges, then such a field is called electrostatic.
Electrostatics studies only electrostatic fields and interactions of fixed charges.
To characterize the electric field, the concept of intensity is introduced
.
tensionu at each point of the electric field is called the vector 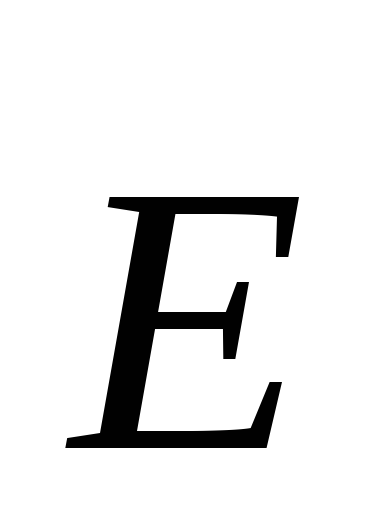 , numerically equal to the ratio of the force with which this field acts on a test positive charge placed in given point, and the magnitude of this charge, and directed in the direction of the force.
, numerically equal to the ratio of the force with which this field acts on a test positive charge placed in given point, and the magnitude of this charge, and directed in the direction of the force.
trial charge, which is introduced into the field, is assumed to be a point and is often called a test charge.
- He does not participate in the creation of the field, which is measured with it.
It is assumed that this charge does not distort the field under study, that is, it is small enough and does not cause a redistribution of the charges that create the field.
If for a test point charge 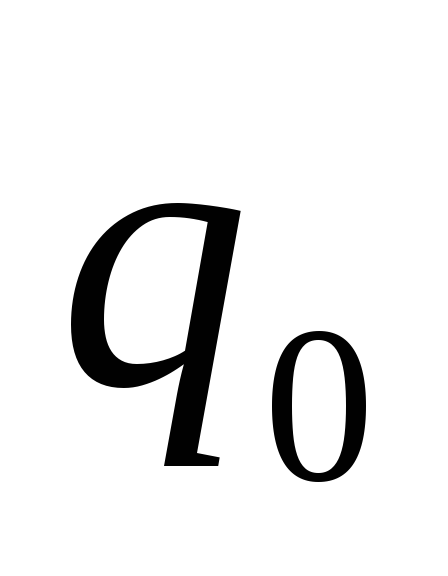 the field acts as a force
the field acts as a force 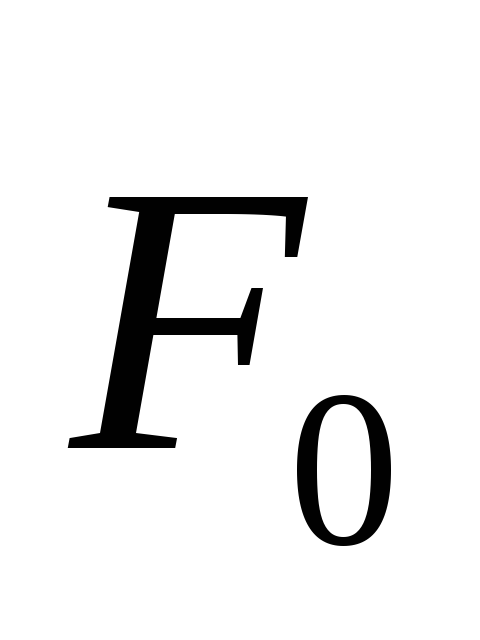 , then the tension
, then the tension 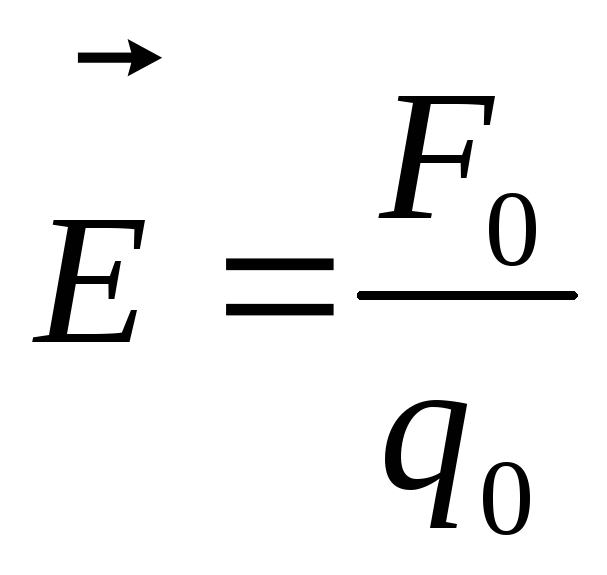 .
.
Tension units:
SI: 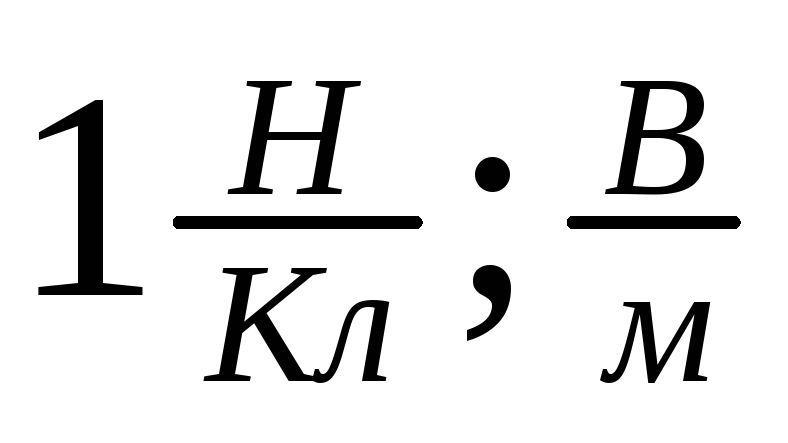
SGSE: 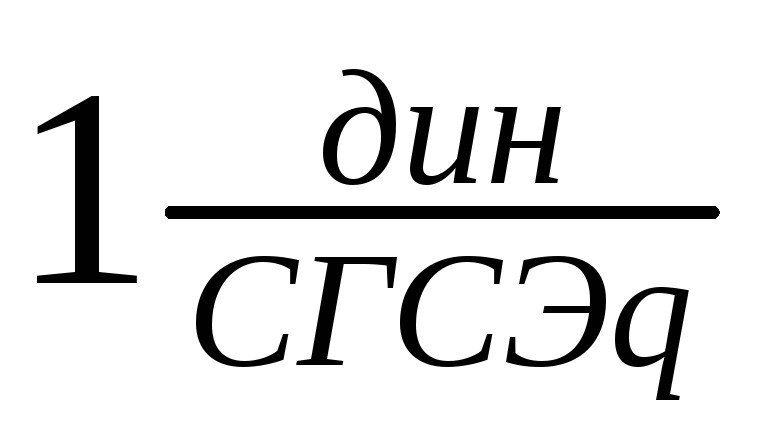
In the SI system expression
for 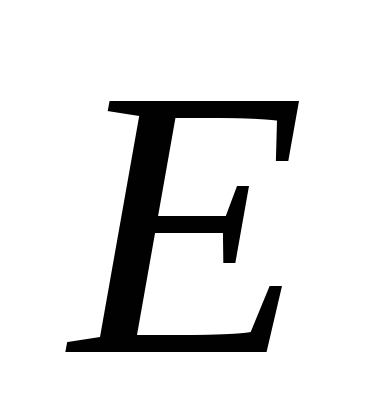 point charge fields:
point charge fields:
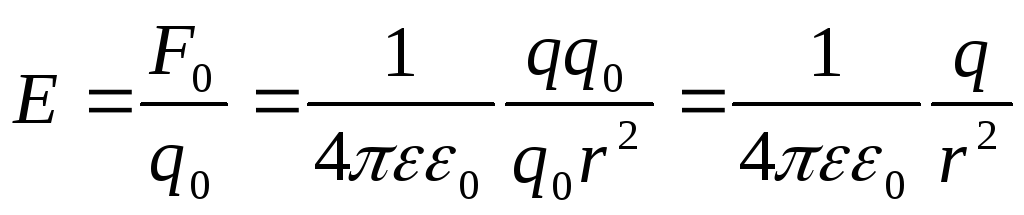 .
.
In vector form: 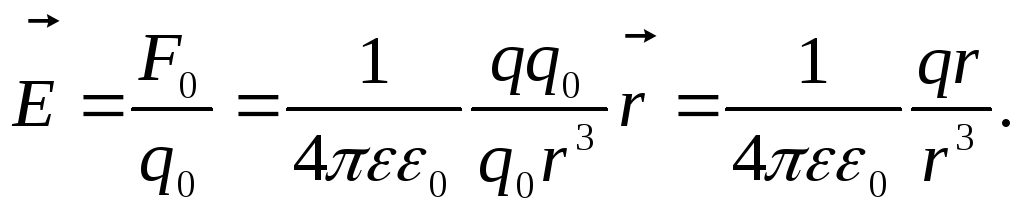
Here  is the radius vector drawn from the charge q, which creates a field, to a given point.
is the radius vector drawn from the charge q, which creates a field, to a given point.
T 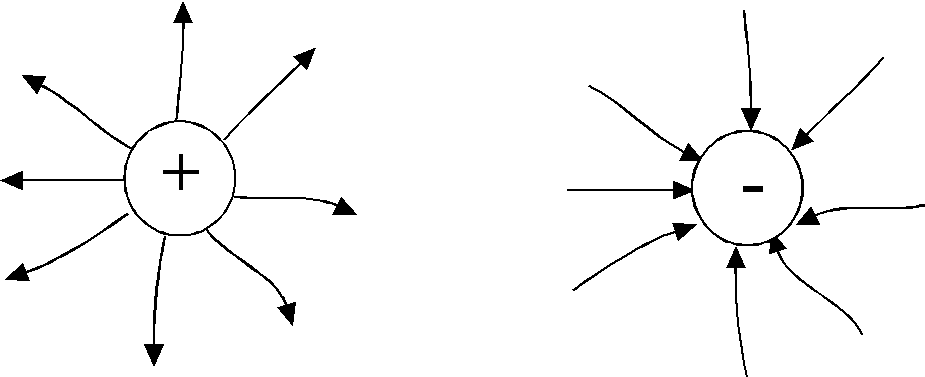 how, electric field strength vectors of a point chargeq
at all points the fields are directed radially(fig.1.3)
how, electric field strength vectors of a point chargeq
at all points the fields are directed radially(fig.1.3)
- from the charge, if it is positive, "source"
- and to the charge if it is negative"stock"
For graphical interpretation electric field is injected the concept of a line of force ortension lines . it
curve , the tangent at each point to which coincides with the intensity vector.
The tension line starts at positive charge and ends in a negative.
The tension lines do not intersect, since at each point of the field the tension vector has only one direction.
Two point charges act on each other with a force that is inversely proportional to the square of the distance between them and directly proportional to the product of their charges (regardless of the sign of the charges)
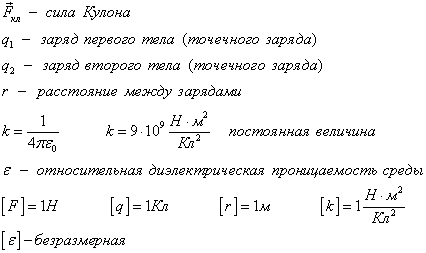
AT various environments, for example, in air and in water, two point charges interact with different forces. The relative permittivity of the medium characterizes this difference. This is a known tabular value. For air.
The constant k is defined as
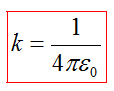
Direction of the Coulomb force
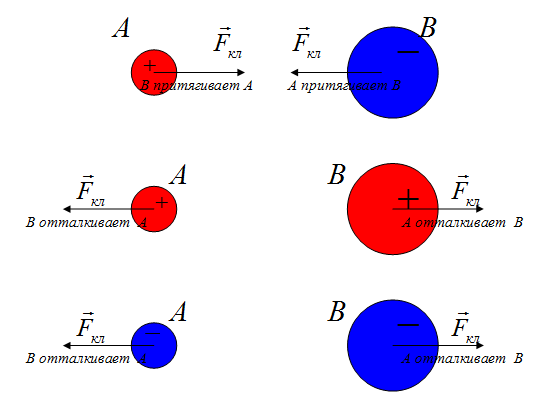
According to Newton's third law, forces of the same nature arise in pairs, equal in magnitude, opposite in direction. If two unequal charges interact, the force with which the larger charge acts on the smaller one (B on A) is equal to the force with which the smaller one acts on the larger one (A on B).
Interestingly, the various laws of physics have some common features. Let's remember the law of gravity. The force of gravity is also inversely proportional to the square of the distance, but already between the masses, and the thought involuntarily arises that this pattern has a deep meaning. Until now, no one has been able to present gravity and electricity as two different manifestations of the same essence.
The force here also varies inversely with the square of the distance, but the difference in the magnitude of electric forces and gravitational forces is striking. In trying to establish the common nature of gravity and electricity, we find such a superiority of electric forces over gravitational forces that it is difficult to believe that both have the same source. How can you say that one is stronger than the other? After all, it all depends on what is the mass and what is the charge. Arguing about how strong gravity acts, you have no right to say: "Let's take a mass of such and such a size," because you choose it yourself. But if we take what Nature herself offers us (her own numbers and measures, which have nothing to do with our inches, years, our measures), then we can compare. We will take an elementary charged particle, such as, for example, an electron. Two elementary particles, two electrons, due to the electric charge, repel each other with a force inversely proportional to the square of the distance between them, and due to gravity, they are attracted to each other again with a force inversely proportional to the square of the distance.
Question: What is the ratio of gravitational force to electrical force? Gravitation is related to electrical repulsion as one is to a number with 42 zeros. This is deeply puzzling. Where could such a huge number come from?
People are looking for this huge factor in other natural phenomena. They go through all sorts big numbers and if you need big number why not take, say, the ratio of the diameter of the Universe to the diameter of a proton - surprisingly, this is also a number with 42 zeros. And they say: maybe this coefficient is equal to the ratio of the diameter of the proton to the diameter of the universe? This is an interesting thought, but as the universe gradually expands, the constant of gravity must also change. Although this hypothesis has not yet been refuted, we do not have any evidence in its favor. On the contrary, some evidence suggests that the constant of gravity did not change in this way. This huge number remains a mystery to this day.
Coulomb's law is a law describing the forces of interaction between point electric charges.
The module of the interaction force of two point charges in vacuum is directly proportional to the product of the modules of these charges and inversely proportional to the square of the distance between them.
Otherwise: Two point charges in vacuum act on each other with forces that are proportional to the product of the modules of these charges, inversely proportional to the square of the distance between them and directed along the straight line connecting these charges. These forces are called electrostatic (Coulomb).
It is important to note that in order for the law to be true, it is necessary:
point charges - that is, the distance between charged bodies is much greater than their sizes - however, it can be proved that the force of interaction of two volumetrically distributed charges with spherically symmetric non-intersecting spatial distributions is equal to the force of interaction of two equivalent point charges located at the centers of spherical symmetry;
their immobility. Otherwise, additional effects take effect: a magnetic field moving charge and the corresponding additional Lorentz force acting on another moving charge;
interaction in vacuum.
However, with some adjustments, the law is also valid for interactions of charges in a medium and for moving charges.
In vector form, in the formulation of S. Coulomb, the law is written as follows:
where is the force with which charge 1 acts on charge 2; - the magnitude of the charges; - radius vector (vector directed from charge 1 to charge 2, and equal, in modulus, to the distance between charges - ); - coefficient of proportionality. Thus, the law indicates that charges of the same name repel (and opposite charges attract).
AT SGSE unit charge is chosen in such a way that the coefficient k is equal to one.
AT International System of Units (SI) one of the basic units is the unit electric current strength ampere, and the unit of charge is pendant is its derivative. The ampere is defined in such a way that k= c 2 10 −7 gn/ m \u003d 8.9875517873681764 10 9 H m 2 / Cl 2 (or Ф −1 m). In SI coefficient k is written as:
where ≈ 8.854187817 10 −12 F/m - electrical constant.
The interaction of electric charges is described by Coulomb's law, which states that the interaction force of two point charges at rest in vacuum is equal to
where the quantity is called an electrical constant, the dimension of the quantity is reduced to the ratio of the dimension of length to the dimension of the electric capacitance (Farad). Electric charges There are two types, which are conventionally called positive and negative. As experience shows, charges attract if they are of the same name and repel if they are of the same name.
Any macroscopic body contains a huge amount of electric charges, since they are part of all atoms: electrons are negatively charged, protons that make up atomic nuclei- positively. However, most of the bodies we are dealing with are not charged, since the number of electrons and protons that make up atoms is the same, and their charges are exactly the same in absolute value. However, bodies can be charged by creating an excess or deficiency of electrons in them compared to protons. To do this, you need to transfer the electrons that are part of a body to another body. Then the first one will have a lack of electrons and, accordingly, a positive charge, the second one will have a negative charge. Such processes occur, in particular, when bodies rub against each other.
If the charges are in a medium that occupies the entire space, then the force of their interaction is weakened compared to the force of their interaction in vacuum, and this weakening does not depend on the magnitude of the charges and the distance between them, but depends only on the properties of the medium. The characteristic of the medium, which shows how many times the force of interaction of charges in this medium is weakened compared to the force of their interaction in vacuum, is called the dielectric constant of this medium and, as a rule, is denoted by the letter. The Coulomb formula in a medium with permittivity takes the form
|
If there are not two, but more point charges, to find the forces acting in this system, a law is used, which is called the principle superposition 1. The principle of superposition states that in order to find the force acting on one of the charges (for example, on a charge) in a system of three point charges, one must do the following. First, you need to mentally remove the charge and, according to Coulomb's law, find the force acting on the charge from the remaining charge. Then you should remove the charge and find the force acting on the charge from the side of the charge. Vector sum received forces and will give the desired strength.
The principle of superposition gives a recipe for finding the interaction force of non-point charged bodies. It is necessary to mentally divide each body into parts that can be considered point parts, according to the Coulomb law, find the strength of their interaction with the point parts into which the second body is divided, sum the resulting vectors. It is clear that such a procedure is mathematically very complicated, if only because it is necessary to add an infinite number of vectors. In mathematical analysis, methods for such summation have been developed, but in school course physics are not included. Therefore, if such a problem occurs, then the summation in it should be easily performed on the basis of certain symmetry considerations. For example, from the described summation procedure it follows that the force acting on a point charge placed at the center of a uniformly charged sphere is equal to zero.
In addition, the student must know (without derivation) the formula for the force acting on a point charge from a uniformly charged sphere and an infinite plane. If there is a sphere of radius , uniformly charged with a charge , and a point charge located at a distance from the center of the sphere, then the magnitude of the interaction force is
if the charge is inside (and not necessarily in the center). From formulas (17.4), (17.5) it follows that the sphere outside creates the same electric field as all its charge placed in the center, and inside - zero.
If there is a very large plane with an area uniformly charged with a charge, and a point charge, then the force of their interaction is equal to
|
where the value 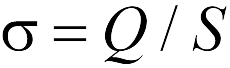 has the meaning of the surface charge density of the plane. As follows from formula (17.6), the force of interaction between a point charge and a plane does not depend on the distance between them. Let us draw the reader's attention to the fact that formula (17.6) is approximate and "works" the more accurately, the farther the point charge is from its edges. Therefore, when formula (17.6) is used, it is often said that it is valid within the framework of neglecting "edge effects", i.e. when the plane is considered infinite.
has the meaning of the surface charge density of the plane. As follows from formula (17.6), the force of interaction between a point charge and a plane does not depend on the distance between them. Let us draw the reader's attention to the fact that formula (17.6) is approximate and "works" the more accurately, the farther the point charge is from its edges. Therefore, when formula (17.6) is used, it is often said that it is valid within the framework of neglecting "edge effects", i.e. when the plane is considered infinite.
Consider now the solution of the data in the first part of the problem book.
According to Coulomb's law (17.1), the magnitude of the interaction force of two charges from tasks 17.1.1 is expressed by the formula
|
Charges repel each other (answer 2 ).
Because a drop of water tasks 17.1.2 has a charge  ( is the charge of the proton), then it has an excess of electrons compared to protons. This means that when three electrons are lost, their excess will decrease, and the droplet charge will become equal to
( is the charge of the proton), then it has an excess of electrons compared to protons. This means that when three electrons are lost, their excess will decrease, and the droplet charge will become equal to  (answer 2
).
(answer 2
).
According to Coulomb's law (17.1), the magnitude of the interaction force of two charges with an increase in the distance between them will decrease by a factor of ( task 17.1.3- answer 4 ).
If the charges of two point bodies are increased by a factor with a constant distance between them, then the force of their interaction, as follows from Coulomb's law (17.1), will increase by a factor ( task 17.1.4- answer 3 ).
With an increase in one charge by 2 times, and the second by 4, the numerator of the Coulomb law (17.1) increases by 8 times, and with an increase in the distance between charges by 8 times, the denominator increases by 64 times. Therefore, the force of interaction of charges from tasks 17.1.5 will decrease by 8 times (answer 4 ).
When the space is filled with a dielectric medium with a dielectric constant = 10, the force of interaction of charges according to the Coulomb law in the medium (17.3) will decrease by 10 times ( task 17.1.6- answer 2 ).
The force of the Coulomb interaction (17.1) acts on both the first and second charges, and since their masses are the same, the accelerations of the charges, as follows from Newton's second law, are the same at any time ( task 17.1.7- answer 3 ).
A similar problem, but the masses of the balls are different. Therefore, with the same force, the acceleration of a ball with a smaller mass is 2 times greater than the acceleration of a ball with a smaller mass. 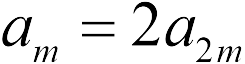 , and this result does not depend on the charges of the balls ( task 17.1.8- answer 2
).
, and this result does not depend on the charges of the balls ( task 17.1.8- answer 2
).
Since the electron is negatively charged, it will be repelled by the ball ( task 17.1.9). But since the initial speed of the electron is towards the ball, it will move in that direction, but its speed will decrease. At some point, it will stop for a moment, and then it will move away from the ball with increasing speed (the answer is 4 ).
In a system of two charged balls connected by a thread ( task 17.1.10), only apply internal forces. Therefore, the system will be at rest, and to find the tension force of the thread, we can use the equilibrium conditions for the balls. Since only the Coulomb force and the thread tension force act on each of them, we conclude from the equilibrium condition that these forces are equal in magnitude.
This value will be equal to the tension force of the threads (the answer 4 ). We note that consideration of the equilibrium condition for the central charge would not help to find the tension force, but would lead to the conclusion that the tension forces of the threads are the same (however, this conclusion is already obvious due to the symmetry of the problem).
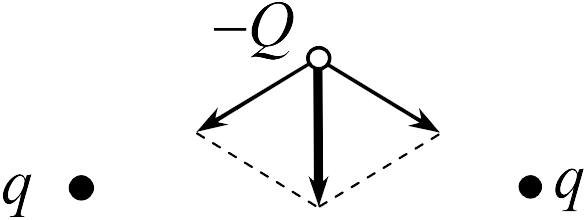 To find the force acting on a charge - in task 17.2.2, we use the principle of superposition. On the charge - the forces of attraction to the left and right charges act (see figure). Since the distances from the charge - to the charges are the same, the modules of these forces are equal to each other and they are directed at the same angles to the straight line connecting the charge - with the middle of the segment -. Therefore, the force acting on the charge is directed vertically downwards (the vector of the resulting force is highlighted in bold in the figure; the answer is 4
).
To find the force acting on a charge - in task 17.2.2, we use the principle of superposition. On the charge - the forces of attraction to the left and right charges act (see figure). Since the distances from the charge - to the charges are the same, the modules of these forces are equal to each other and they are directed at the same angles to the straight line connecting the charge - with the middle of the segment -. Therefore, the force acting on the charge is directed vertically downwards (the vector of the resulting force is highlighted in bold in the figure; the answer is 4
).
(answer 3 ).
From formula (17.6) we conclude that the correct answer in task 17.2.5 - 4 . AT task 17.2.6 you need to use the formula for the interaction force of a point charge and a sphere (formulas (17.4), (17.5)). We have = 0 (answer 3 ).
The basic law of interaction of electric charges was found by Charles Coulomb in 1785 experimentally. Coulomb found that the force of interaction between two small charged metal balls is inversely proportional to the square of the distance 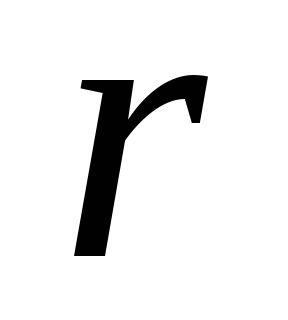 between them and depends on the magnitude of the charges
between them and depends on the magnitude of the charges 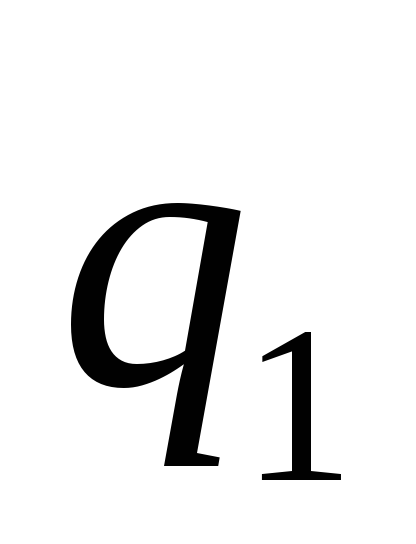 and
and 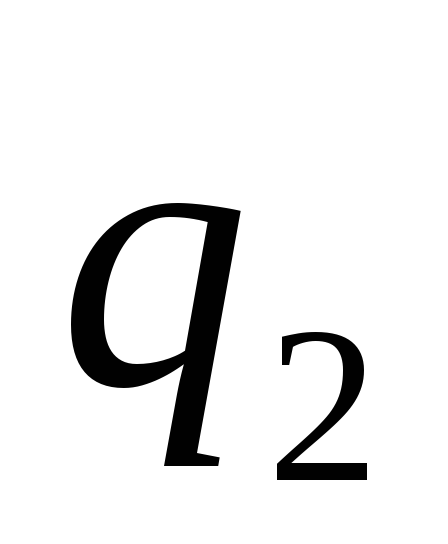 :
:
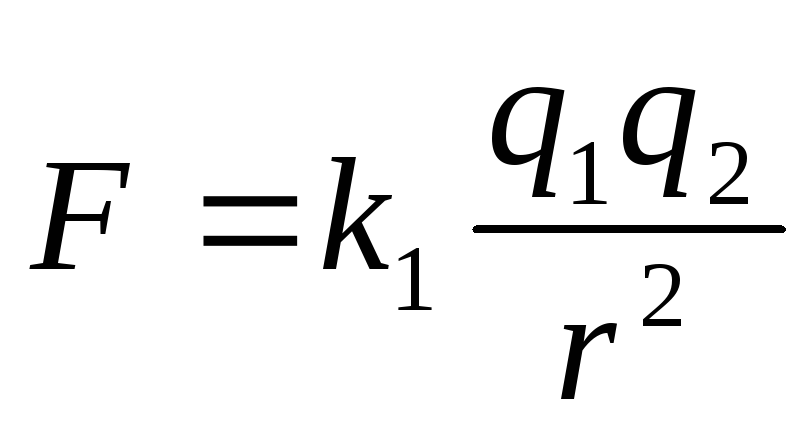 ,
,
where 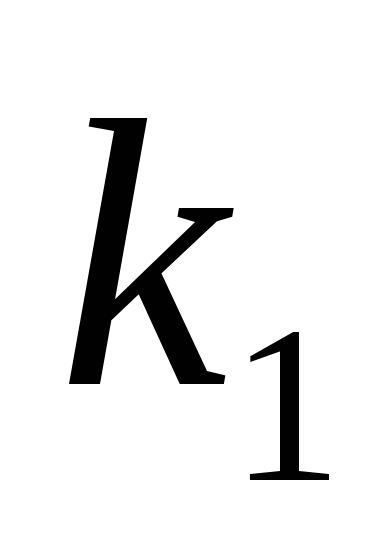 -proportionality factor
-proportionality factor
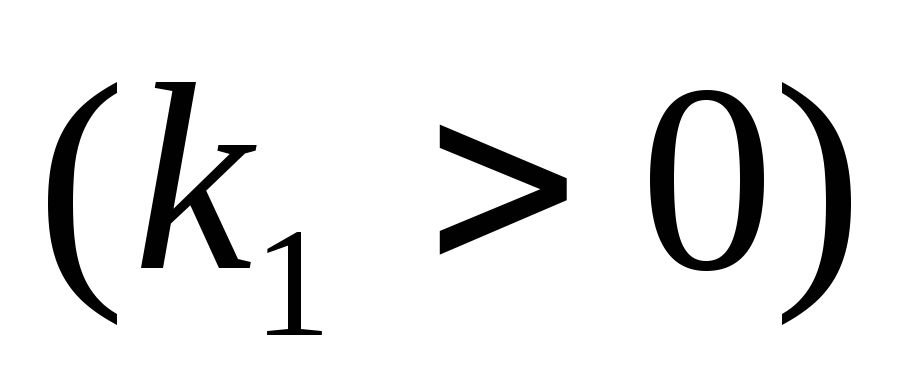 .
.
Forces acting on charges, are central
, that is, they are directed along the straight line connecting the charges. 
Coulomb's law can be written in vector form: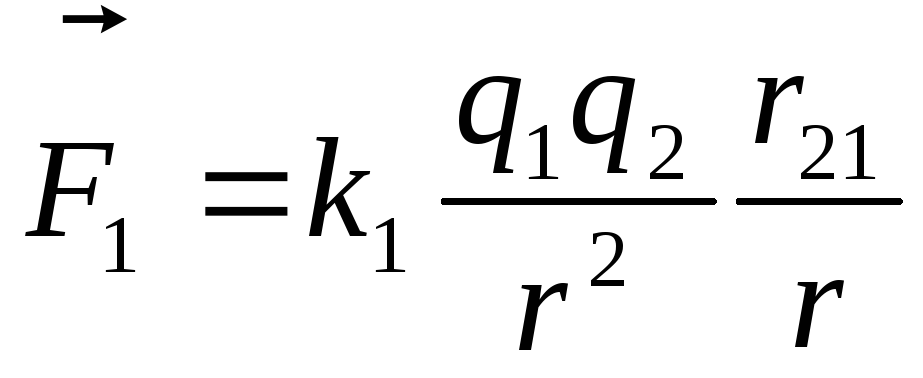 ,
,
where 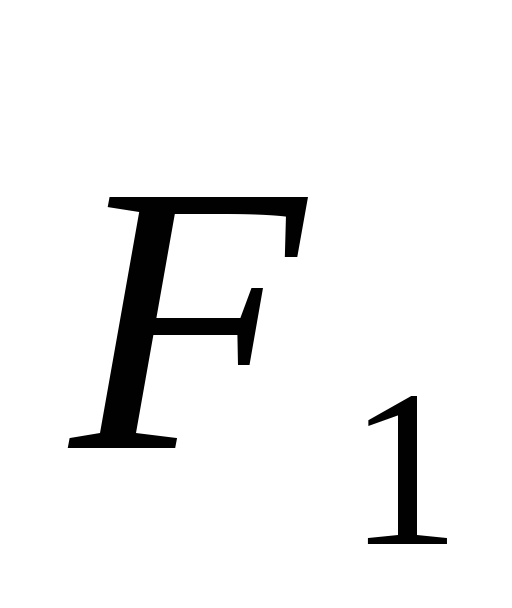 -
-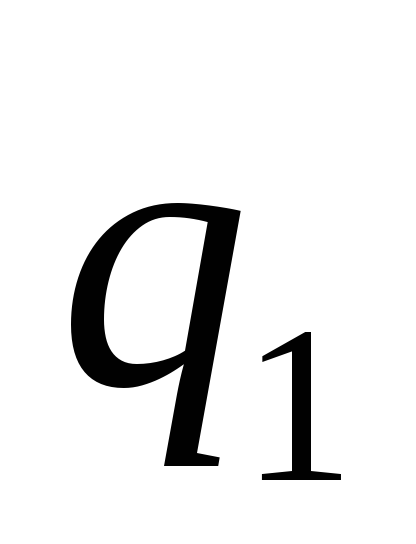 charge side
charge side 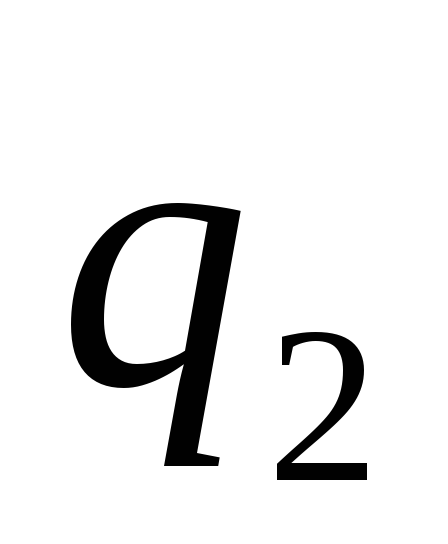 ,
,
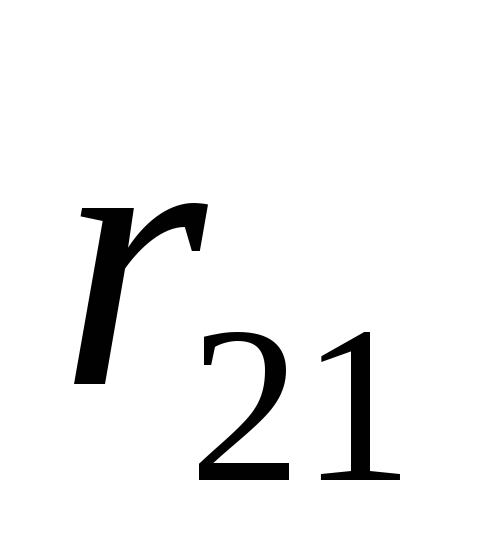 is the radius vector connecting the charge
is the radius vector connecting the charge 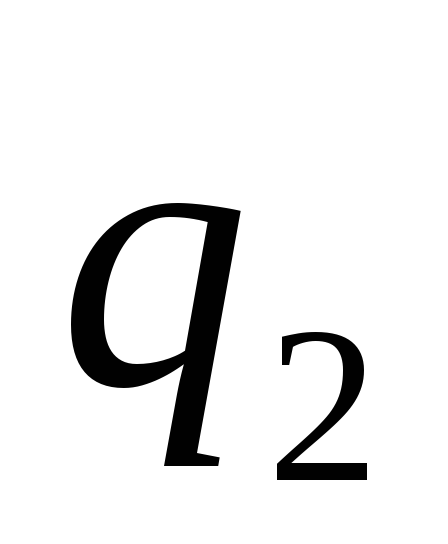 with charge
with charge 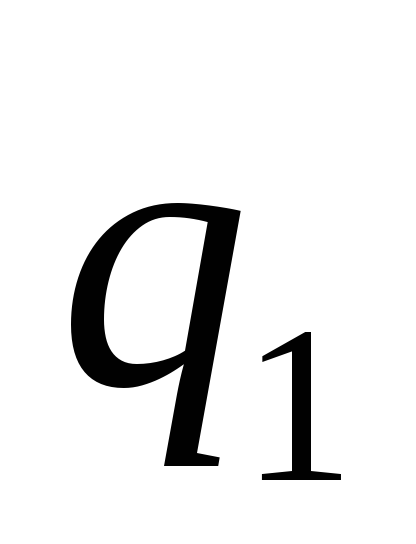 ;
;
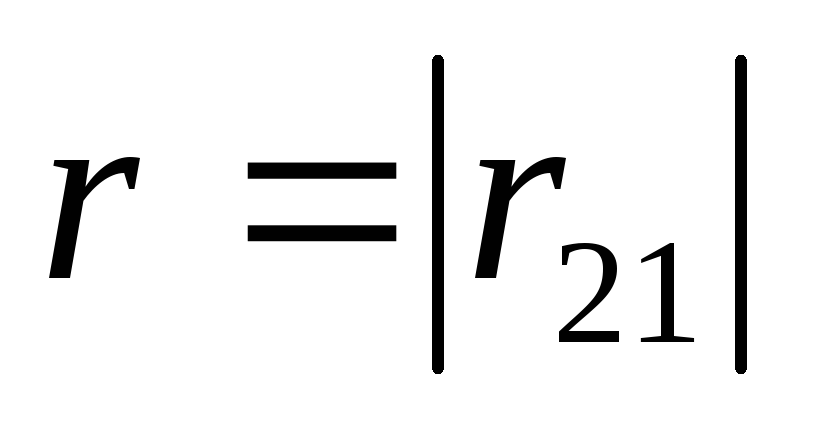 is the modulus of the radius vector.
is the modulus of the radius vector.
Force acting on a charge 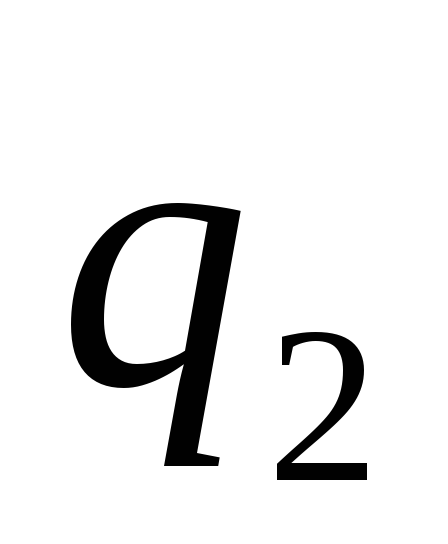 from the side
from the side 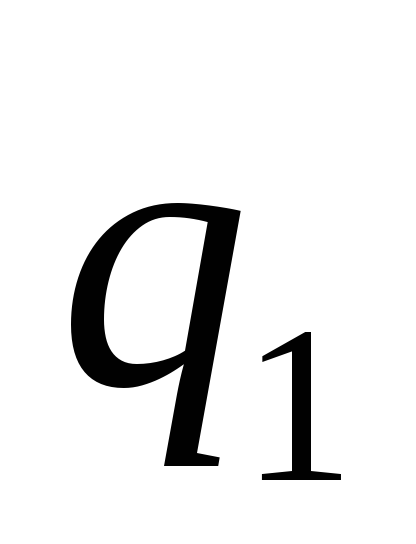 is equal to
is equal to 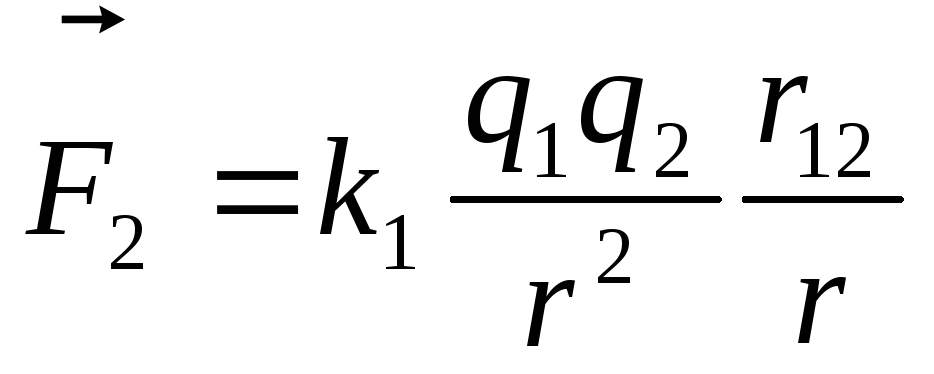 ,
, .
.
Coulomb's law in this form
fair only for the interaction of point electric charges, that is, such charged bodies, the linear dimensions of which can be neglected in comparison with the distance between them.
expresses the strength of the interaction between fixed electric charges, that is, this is the electrostatic law.
Formulation of Coulomb's law:
The strength of the electrostatic interaction between two point electric charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between them.
Proportionality factor
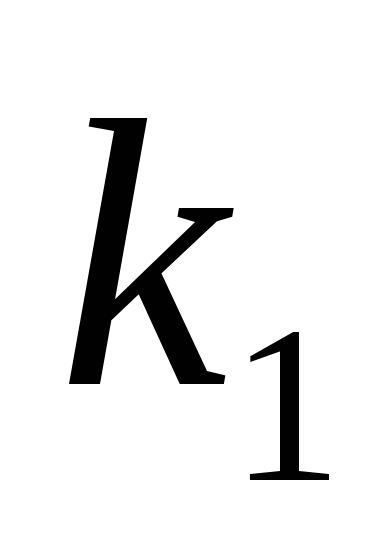 in Coulomb's law depends
in Coulomb's law depends
from the properties of the environment
selection of units of measure for the quantities included in the formula.
That's why 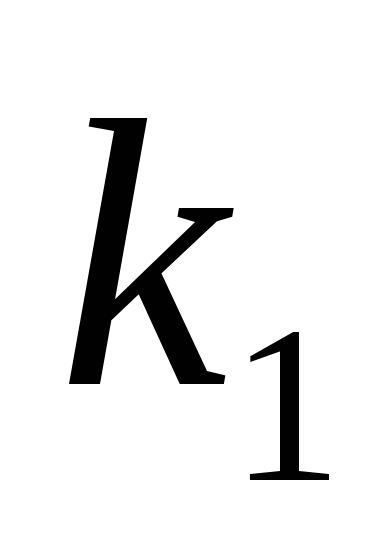 can be represented by the relation
can be represented by the relation 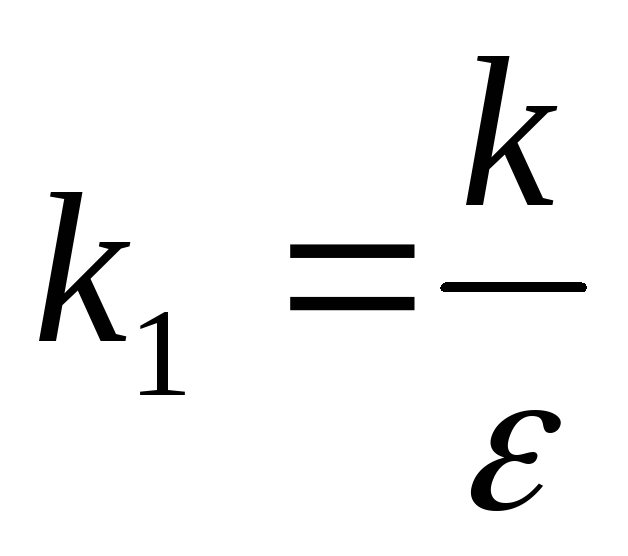 ,
,
where  -coefficient depending only on the choice of system of units;
-coefficient depending only on the choice of system of units;
 - a dimensionless quantity characterizing the electrical properties of the medium is called relative permittivity of the medium
. It does not depend on the choice of the system of units and is equal to one in vacuum.
- a dimensionless quantity characterizing the electrical properties of the medium is called relative permittivity of the medium
. It does not depend on the choice of the system of units and is equal to one in vacuum.
Then Coulomb's law takes the form: 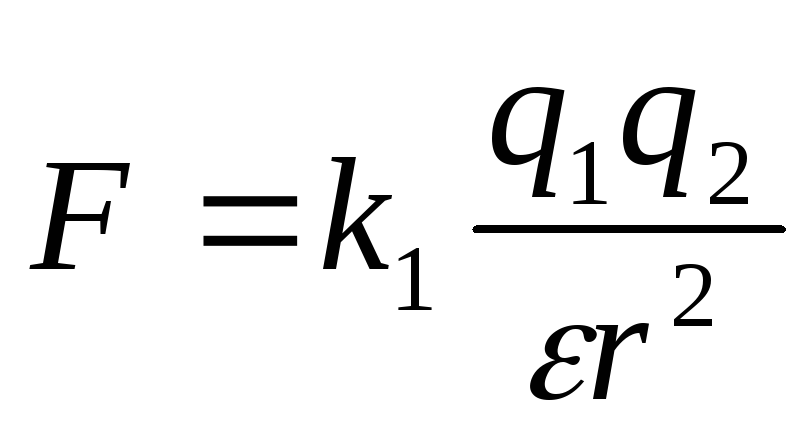 ,
,
for vacuum 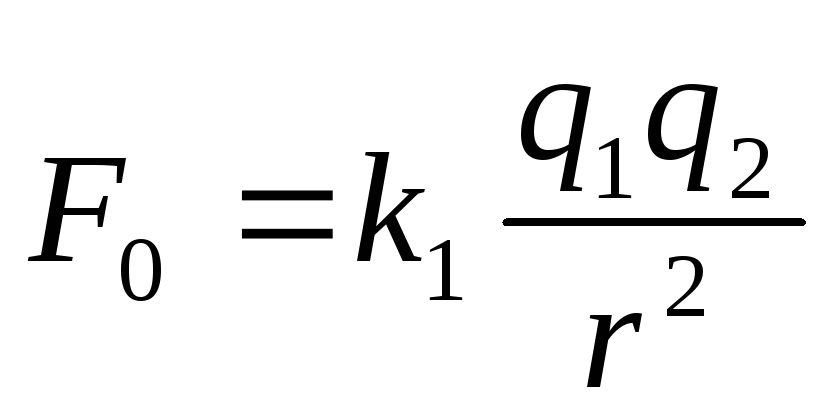 ,
,
then 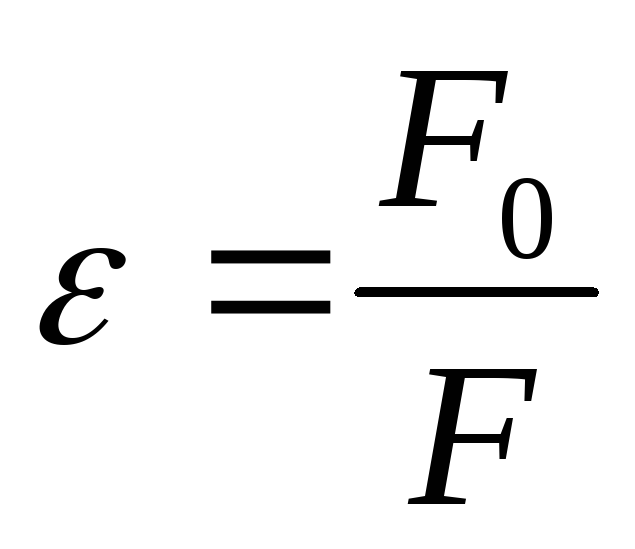 -the relative permittivity of a medium shows how many times in a given medium the force of interaction between two point electric charges
-the relative permittivity of a medium shows how many times in a given medium the force of interaction between two point electric charges 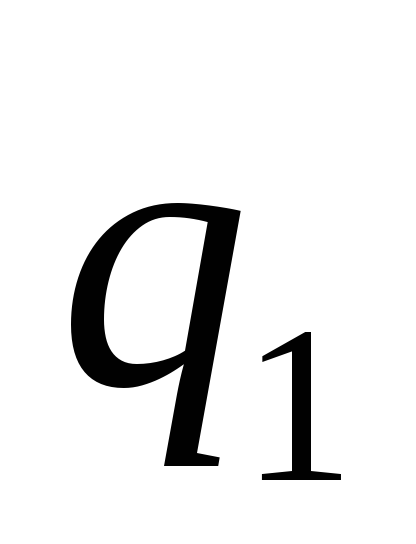 and
and 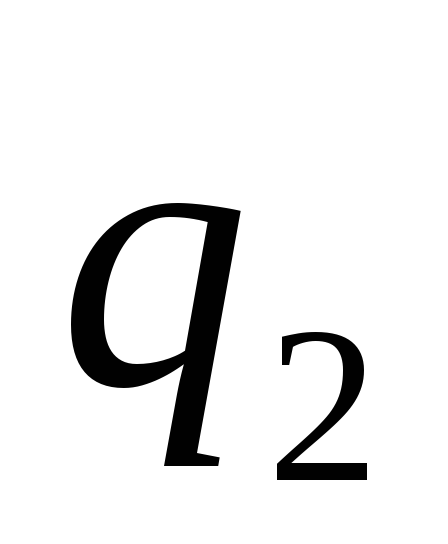 , located at a distance from each other
, located at a distance from each other 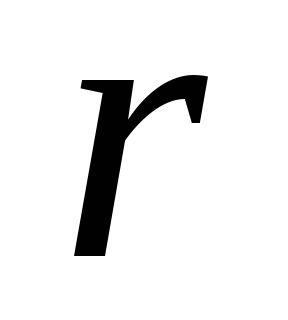 , less than in vacuum.
, less than in vacuum.
In the SI system coefficient 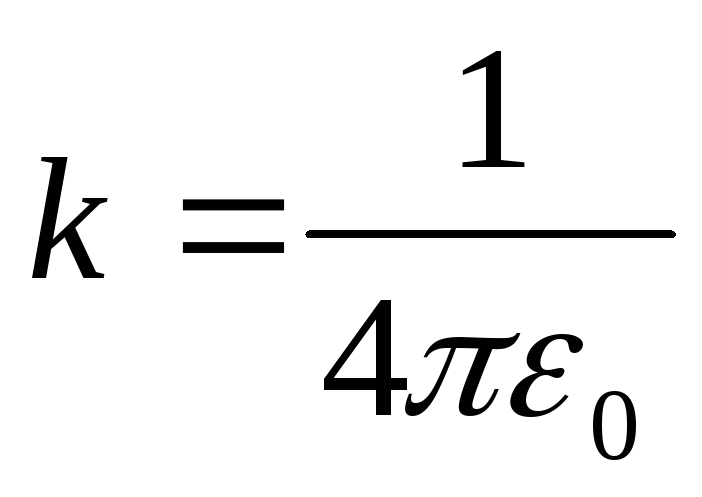 , and
, and
Coulomb's law has the form: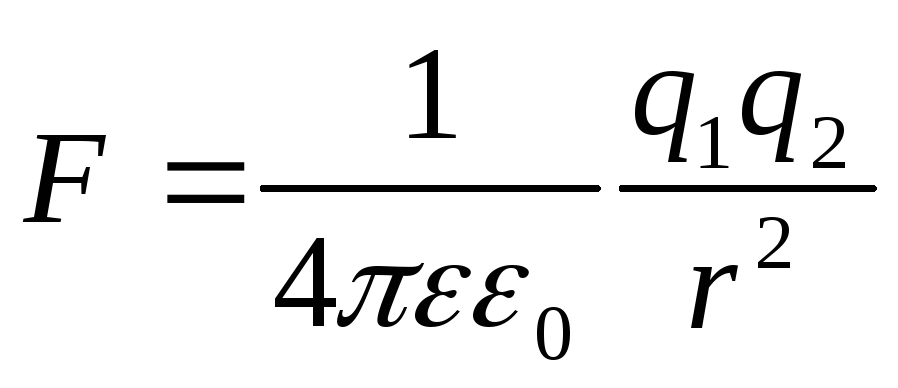 .
.
it rationalized notation of the law K oolon.
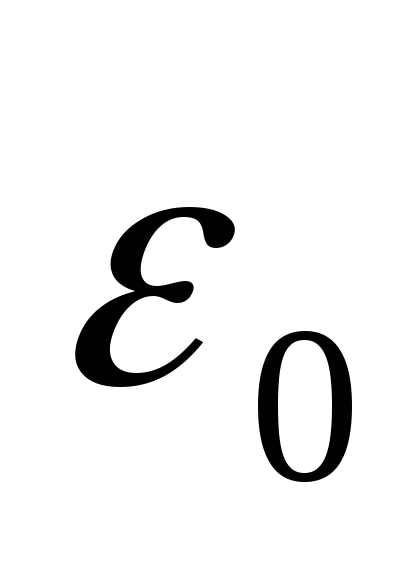 - electrical constant,
- electrical constant, 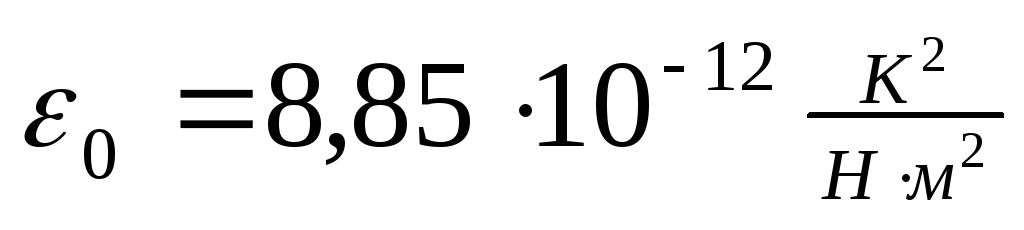 .
.
In the GSSE system
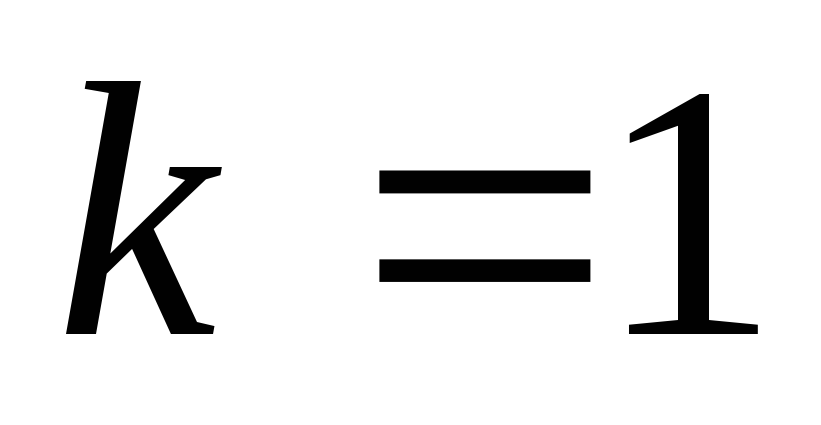 ,
,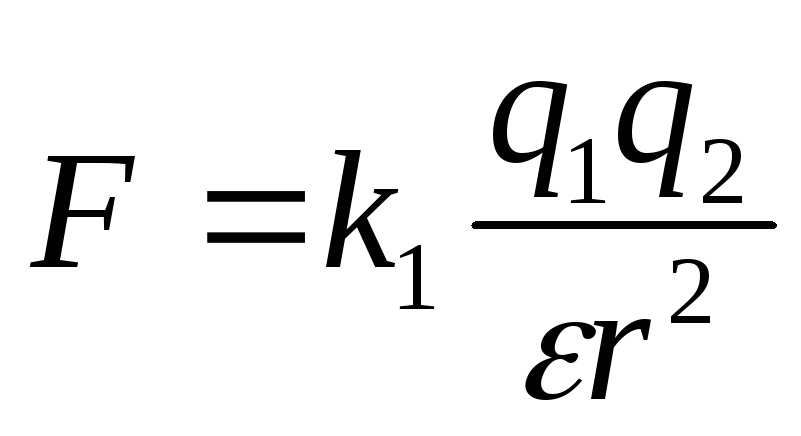 .
.
In vector form, Coulomb's law takes the form 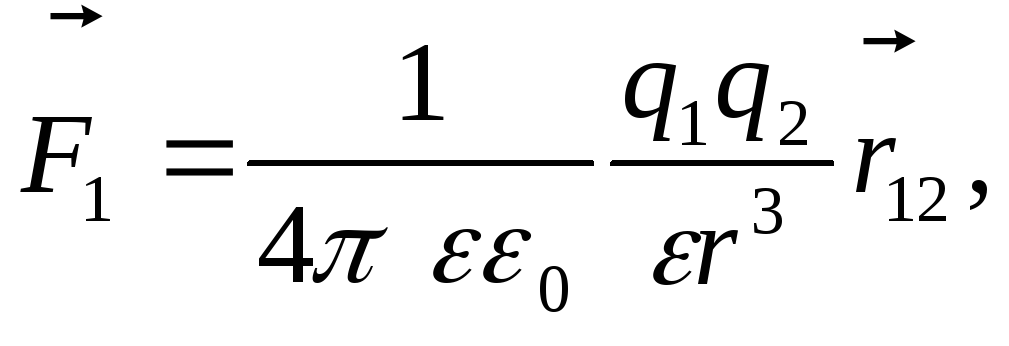
where 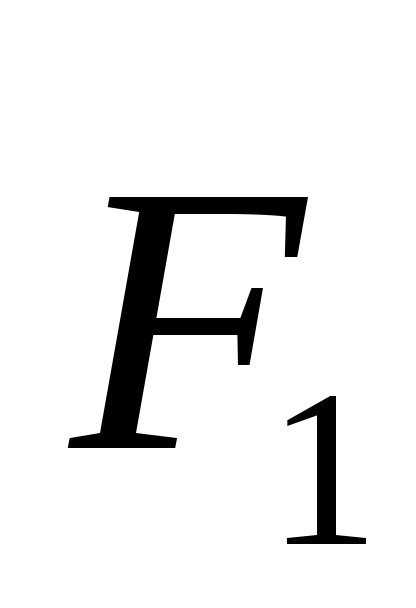 -the vector of the force acting on the charge
-the vector of the force acting on the charge 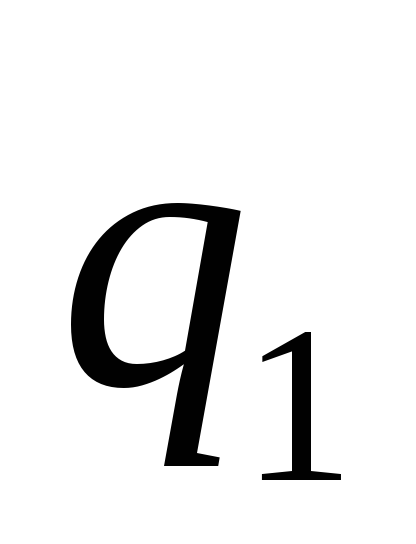 charge side
charge side
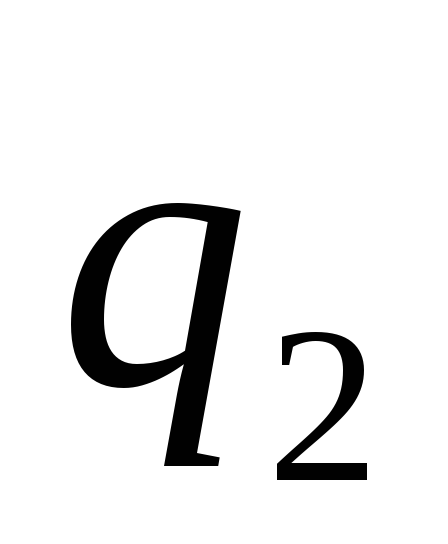 ,
,
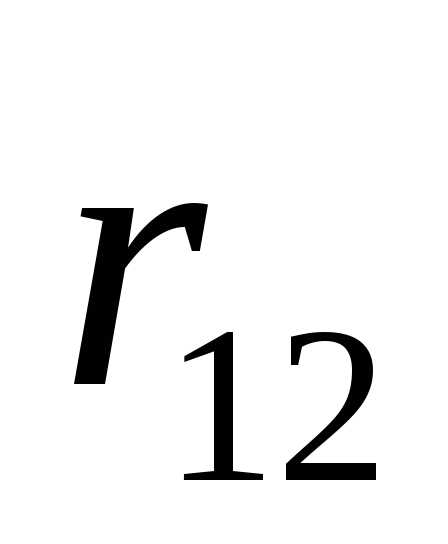
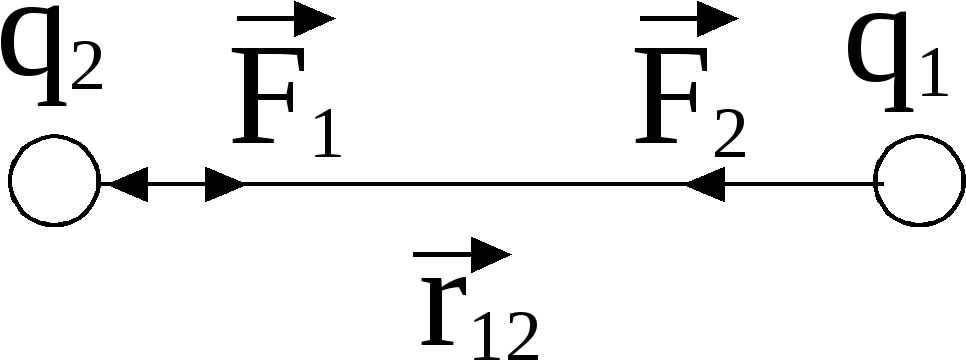 is the radius vector connecting the charge
is the radius vector connecting the charge 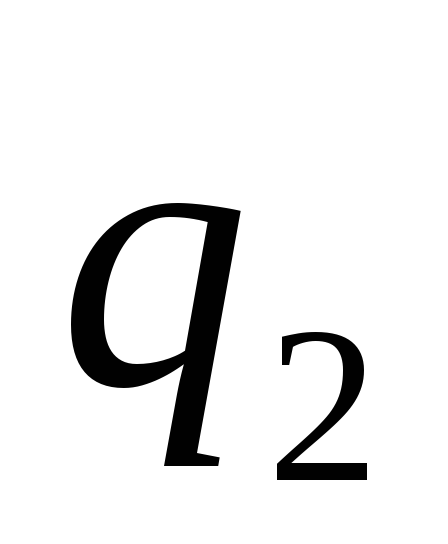 with charge
with charge
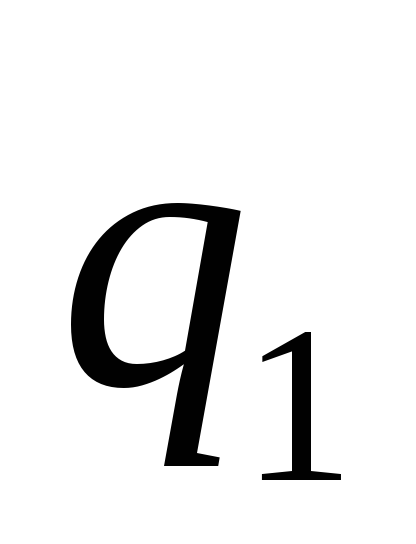
r is the modulus of the radius vector 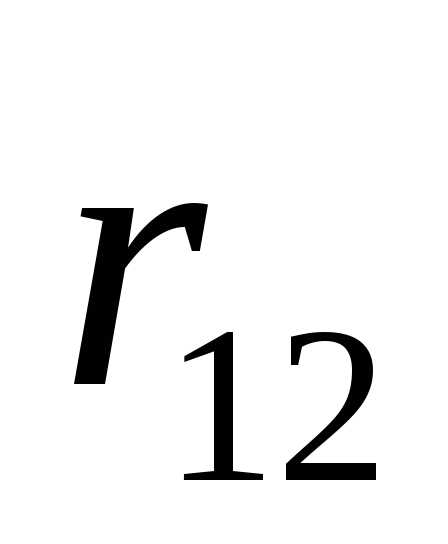 .
.
Any charged body consists of many point electric charges, so the electrostatic force with which one charged body acts on another is equal to the vector sum of the forces applied to all point charges of the second body from each point charge of the first body.
1.3 Electric field. Tension.
Space, in which there is an electric charge, has certain physical properties.
For everyone another the charge introduced into this space is acted upon by electrostatic Coulomb forces.
If a force acts at every point in space, then we say that there is a force field in this space.
The field, along with matter, is a form of matter.
If the field is stationary, that is, does not change in time, and is created by stationary electric charges, then such a field is called electrostatic.
Electrostatics studies only electrostatic fields and interactions of fixed charges.
To characterize the electric field, the concept of intensity is introduced
.
tensionu at each point of the electric field is called the vector  , numerically equal to the ratio of the force with which this field acts on a test positive charge placed at a given point, and the magnitude of this charge, and directed in the direction of the force.
, numerically equal to the ratio of the force with which this field acts on a test positive charge placed at a given point, and the magnitude of this charge, and directed in the direction of the force.
trial charge, which is introduced into the field, is assumed to be a point and is often called a test charge.
- He does not participate in the creation of the field, which is measured with it.
It is assumed that this charge does not distort the field under study, that is, it is small enough and does not cause a redistribution of the charges that create the field.
If for a test point charge 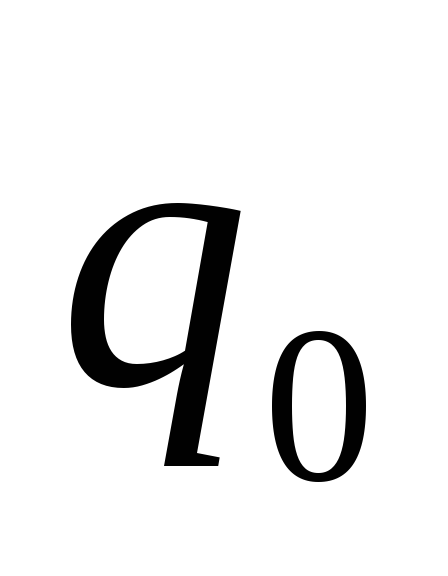 the field acts as a force
the field acts as a force 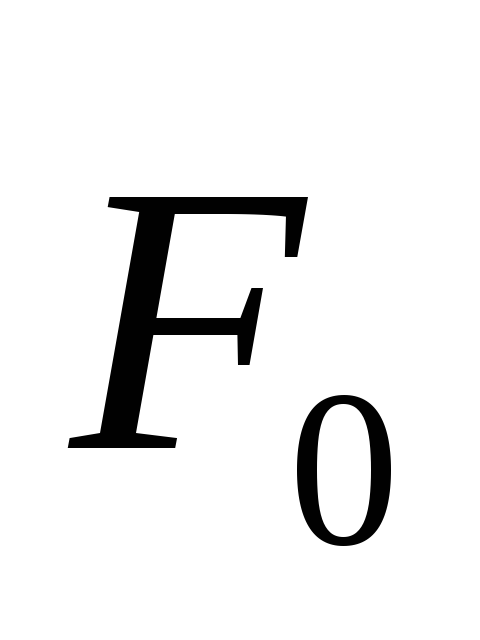 , then the tension
, then the tension 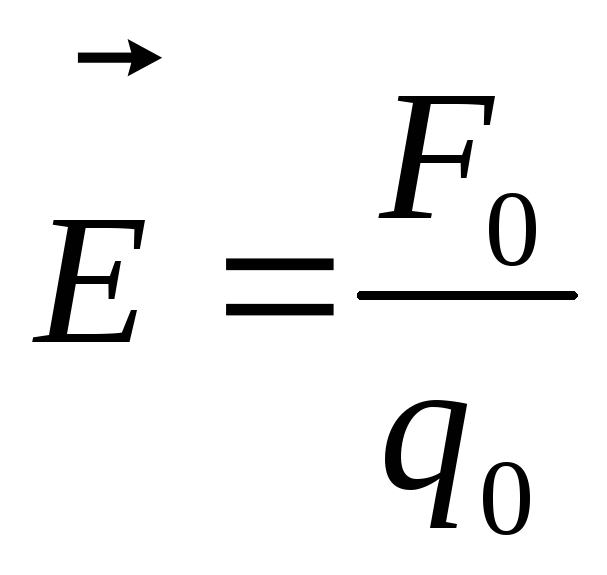 .
.
Tension units:
SI: 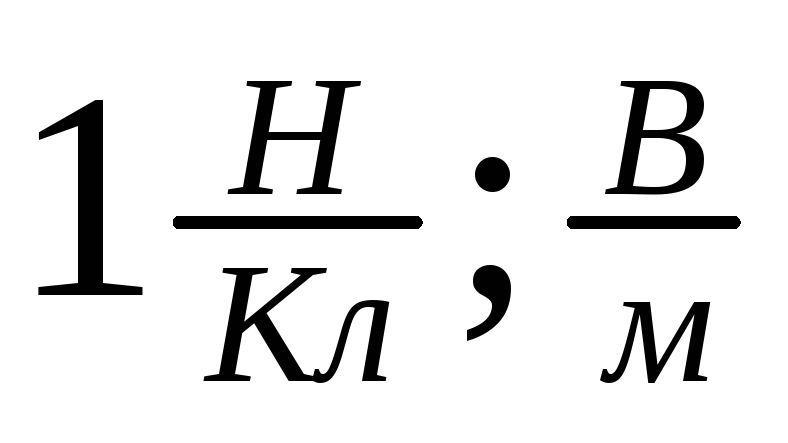
SGSE: 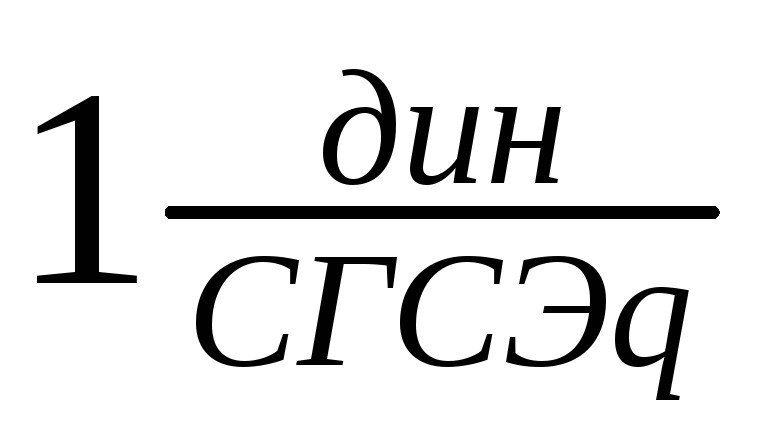
In the SI system expression
for  point charge fields:
point charge fields:
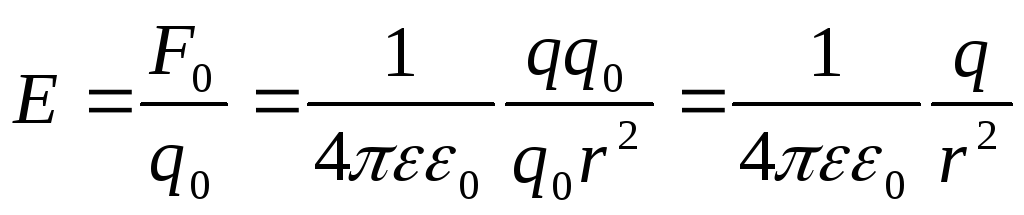 .
.
In vector form: 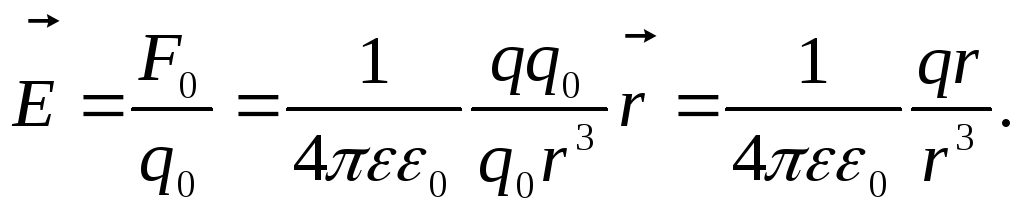
Here 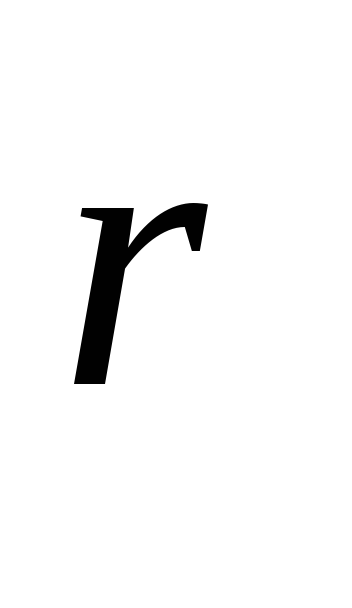 is the radius vector drawn from the charge q, which creates a field, to a given point.
is the radius vector drawn from the charge q, which creates a field, to a given point.
T  how, electric field strength vectors of a point chargeq
at all points the fields are directed radially(fig.1.3)
how, electric field strength vectors of a point chargeq
at all points the fields are directed radially(fig.1.3)
- from the charge, if it is positive, "source"
- and to the charge if it is negative"stock"
For graphical interpretation electric field is injected the concept of a line of force ortension lines . it
curve , the tangent at each point to which coincides with the intensity vector.
The tension line starts on a positive charge and ends on a negative one.
The tension lines do not intersect, since at each point of the field the tension vector has only one direction.