Neutrons have mass and charge. Neutron (elementary particle)
Neutron ( elementary particle)
This article was written by Vladimir Gorunovich for the site "Wikiknowledge", placed on this site in order to protect information from vandals, and then supplemented on this site.
The field theory of elementary particles, acting within the framework of SCIENCE, relies on a foundation proven by PHYSICS:
- classical electrodynamics,
- quantum mechanics,
- Conservation laws are the fundamental laws of physics.
Using elementary particles that do not exist in nature, inventing fundamental interactions that do not exist in nature, or replacing the interactions that exist in nature with fabulous ones, ignoring the laws of nature, doing mathematical manipulations on them (creating the appearance of science) - this is the lot of FAIRY TALES masquerading as science. As a result, physics slipped into the world of mathematical fairy tales.
1 Neutron radius
2 Magnetic moment of the neutron
3 Neutron electric field
4 Neutron rest mass
5 Neutron lifetime
6 New Physics: Neutron (elementary particle) - result
Neutron - elementary particle quantum number L=3/2 (spin = 1/2) - baryon group, proton subgroup, electric charge +0 (systematization by field theory elementary particles).
According to the field theory of elementary particles (a theory built on a scientific foundation and the only one that received the correct spectrum of all elementary particles), the neutron consists of a rotating polarized alternating electro magnetic field with a constant component. All the unsubstantiated assertions of the Standard Model that the neutron supposedly consists of quarks have nothing to do with reality. - Physics has experimentally proven that the neutron has electromagnetic fields (zero value of the total electric charge, does not yet mean the absence of a dipole electric field, which even the Standard Model indirectly had to admit, by introducing electric charges for the elements of the neutron structure), and also a gravitational field. The fact that elementary particles do not just possess - but consist of electromagnetic fields, physics brilliantly guessed 100 years ago, but it was not possible to build a theory until 2010. Now, in 2015, the theory of gravity of elementary particles also appeared, which established the electromagnetic nature of gravity and received the equations of the gravitational field of elementary particles, different from the equations of gravity, on the basis of which more than one mathematical fairy tale in physics was built.
The structure of the electromagnetic field of the neutron (E-constant electric field, H-constant magnetic field, yellow a variable electromagnetic field is noted).
Energy balance (percentage of total internal energy):
- constant electric field (E) - 0.18%,
- permanent magnetic field (H) - 4.04%,
- alternating electromagnetic field - 95.78%.
Despite the zero electric charge, the neutron has a dipole electric field.
1 Neutron radius
The field theory of elementary particles defines the radius (r) of an elementary particle as the distance from the center to the point where the maximum mass density is reached. 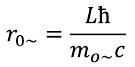
For a neutron, this will be 3.3518 ∙ 10 -16 m. To this we must add the thickness of the electromagnetic field layer 1.0978 ∙ 10 -16 m. 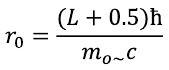
Then it will be 4.4496 ∙10 -16 m. Thus, the outer boundary of the neutron should be at a distance of more than 4.4496 ∙10 -16 m from the center. The result is a value almost equal to the radius of the proton, and this is not surprising. The radius of an elementary particle is determined quantum number L and the value of the rest mass. Both particles have the same set of quantum numbers L and M L , and the rest masses differ slightly.
2 Magnetic moment of the neutron
A counterweight quantum theory The field theory of elementary particles states that the magnetic fields of elementary particles are not created by the spin rotation of electric charges, but exist simultaneously with a constant electric field as a constant component of the electromagnetic field. Therefore, all elementary particles with quantum number L>0 have magnetic fields.
The field theory of elementary particles does not consider the magnetic moment of the neutron to be anomalous - its value is determined by a set of quantum numbers to the extent that quantum mechanics works in an elementary particle.
So the magnetic moment of the neutron is created by the current:
- (0) with magnetic moment -1 eħ/m 0n c
3 Neutron electric field
Despite the zero electric charge, according to the field theory of elementary particles, the neutron must have a constant electric field. The electromagnetic field that makes up the neutron has a constant component, and, therefore, the neutron must have a constant magnetic field and a constant electric field. Since the electric charge is zero, the constant electric field will be dipole. That is, the neutron must have a constant electric field similar to the field of two distributed parallel electric charges of equal magnitude and opposite sign. At large distances, the electric field of the neutron will be practically imperceptible due to the mutual compensation of the fields of both charge signs. But at distances of the order of the neutron radius, this field will have a significant effect on interactions with other elementary particles of similar sizes. First of all, this concerns the interaction in atomic nuclei neutron with proton and neutron with neutron. For neutron - neutron interaction, these will be repulsive forces with the same direction of spins and attractive forces with the opposite direction of spins. For the neutron - proton interaction, the sign of the force depends not only on the orientation of the spins, but also on the displacement between the planes of rotation of the electromagnetic fields of the neutron and proton.
So, the neutron must have a dipole electric field of two distributed parallel symmetric ring electric charges (+0.75e and -0.75e), of average radius ![]() located at a distance
located at a distance ![]()
The electric dipole moment of the neutron (according to the field theory of elementary particles) is equal to: ![]()
where ħ is Planck's constant, L is the main quantum number in the field theory of elementary particles, e is the elementary electric charge, m 0 is the rest mass of the neutron, m 0~ is the rest mass of the neutron enclosed in an alternating electromagnetic field, c is the speed of light, P - electric dipole moment vector (perpendicular to the neutron plane, passes through the center of the particle and directed towards the positive electric charge), s - average distance between charges, r e - electric radius of the elementary particle.
As you can see, electric charges are close in magnitude to the charges of the supposed quarks (+2/3e=+0.666e and -2/3e=-0.666e) in the neutron, but unlike quarks, electromagnetic fields exist in nature, and a similar structure of constant any neutral elementary particle has an electric field, regardless of the size of the spin and... .
The potential of the neutron electric dipole field at point (A) (in the near zone 10s > r > s approximately), in the SI system is:
where θ is the angle between the dipole moment vector P and direction to the observation point A, r 0 - normalization parameter equal to r 0 =0.8568Lħ/(m 0~ c), ε 0 - electrical constant, r - distance from the axis (rotation of the alternating electromagnetic field) of the elementary particle to the observation point A, h is the distance from the plane of the particle (passing through its center) to the observation point A, h e is the average height of the electric charge in a neutral elementary particle (equal to 0.5s), |...| is the modulus of the number, P n is the magnitude of the vector P n. (There is no multiplier in the CGS system.)
The strength E of the neutron electric dipole field (in the near zone 10s > r > s approximately), in the SI system is:
where n=r/|r| - a unit vector from the center of the dipole in the direction of the observation point (A), the dot (∙) denotes the scalar product, the vectors are in bold. (There is no multiplier in the CGS system.)
Components of the electric dipole field strength of the neutron (in the near zone 10s>r>s approximately) longitudinal (| |) (along the radius vector drawn from the dipole to given point) and transverse (_|_) in the SI system:
Where θ is the angle between the direction of the dipole moment vector P n and the radius vector to the point of observation (there is no multiplier in the CGS system).
The third component of the electric field strength is orthogonal to the plane in which the dipole moment vector lies P n of the neutron and the radius vector, - is always equal to zero.
The potential energy U of the interaction of the electric dipole field of the neutron (n) with the electric dipole field of another neutral elementary particle (2) at the point (A) in the far zone (r>>s), in the SI system is equal to:
where θ n2 is the angle between the vectors of electric dipole moments P n and P 2 , θ n is the angle between the dipole vector electric moment P n and vector r, θ 2 - the angle between the vector of the dipole electric moment P 2 and vector r, r- a vector from the center of the dipole electric moment p n to the center of the dipole electric moment p 2 (to the observation point A). (There is no multiplier in the CGS system)
The normalization parameter r 0 is introduced in order to reduce the deviation of the value of E from that calculated using classical electrodynamics and integral calculus in the near zone. Normalization occurs at a point lying in a plane parallel to the plane of the neutron, remote from the center of the neutron at a distance (in the plane of the particle) and with a height shift of h=ħ/2m 0~ c, where m 0~ is the value of the mass enclosed in an alternating electromagnetic field resting neutron (for a neutron m 0~ = 0.95784 m. For each equation, the parameter r 0 is calculated independently. As an approximate value, you can take the field radius:
From all of the above, it follows that the electric dipole field of the neutron (the existence of which in nature, the physics of the 20th century did not even know), according to the laws of classical electrodynamics, will interact with charged elementary particles.
4 Neutron rest mass
In accordance with classical electrodynamics and Einstein's formula, the rest mass of elementary particles with quantum number L>0, including the neutron, is defined as the energy equivalent of their electromagnetic fields: ![]()
where the definite integral is taken over the entire electromagnetic field of the elementary particle, E is the electric field strength, H is the magnetic field strength. Here all components of the electromagnetic field are taken into account: a constant electric field (which the neutron has), a constant magnetic field, an alternating electromagnetic field. This small, but very capacious formula for physics, on the basis of which the equations of the gravitational field of elementary particles are obtained, will send to the scrap more than one fabulous "theory" - therefore, some of their authors will hate it.
As follows from the above formula, the value of the rest mass of the neutron depends on the conditions in which the neutron is. So by placing a neutron in a constant external electric field (for example, an atomic nucleus), we will affect E 2, which will affect the mass of the neutron and its stability. A similar situation will arise when a neutron is placed in a constant magnetic field. Therefore, some properties of a neutron inside an atomic nucleus differ from the same properties of a free neutron in vacuum, far from the fields.
5 Neutron lifetime
The lifetime of 880 seconds, established by physics, corresponds to a free neutron.
The field theory of elementary particles states that the lifetime of an elementary particle depends on the conditions in which it is located. By placing a neutron in an external field (for example, magnetic) we change the energy contained in its electromagnetic field. You can choose the direction of the external field so that internal energy neutron has decreased. As a result, less energy will be released during the decay of a neutron, which will complicate the decay and increase the lifetime of an elementary particle. It is possible to choose such a value of the external field strength that the decay of the neutron will require additional energy and, consequently, the neutron will become stable. This is exactly what is observed in atomic nuclei (for example, deuterium), in which the magnetic field of neighboring protons does not allow the decay of neutrons in the nucleus. On the other hand, when additional energy is introduced into the nucleus, neutron decays can again become possible.
6 New Physics: Neutron (elementary particle) - result
The Standard Model (omitted from this article, but claimed to be true in the 20th century) states that the neutron is a bound state of three quarks: one "up" (u) and two "down" (d) quarks (assumed quark structure of the neutron: udd ). Since the presence of quarks in nature has not been experimentally proven, an electric charge equal in magnitude to the charge of hypothetical quarks has not been found in nature, and there are only indirect evidence that can be interpreted as the presence of traces of quarks in some interactions of elementary particles, but can also be interpreted differently, then the statement The Standard Model that the neutron has a quark structure remains just an unproven assumption. Any model, including the Standard one, has the right to assume any structure of elementary particles, including the neutron, but until the corresponding particles that allegedly consist of the neutron are found at accelerators, the statement of the model should be considered unproven.
The Standard Model, describing the neutron, introduces quarks with gluons that are not found in nature (no one has found gluons either), fields and interactions that do not exist in nature, and conflicts with the law of conservation of energy;
Field theory of elementary particles ( New physics) describes the neutron based on the fields and interactions existing in nature within the framework of laws operating in nature - this is SCIENCE.
Vladimir Gorunovich
Page 1
The neutron charge is zero. Consequently, neutrons do not play a role in the magnitude of the charge of the nucleus of an atom. The serial number of chromium is equal to the same value.
Proton charge qp e Neutron charge is equal to zero.
It is easy to see that in this case the charge of the neutron is zero, and that of the proton is 1, as expected. All the baryons included in two families are obtained - the eight and the ten. Mesons are made up of a quark and an antiquark. The bar denotes antiquarks; their electric charge differs in sign from that of the corresponding quark. A strange quark does not enter into a pi-meson, pi-mesons, as we have already said, are particles with strangeness and spin equal to zero.
Since the charge of the proton equal to the charge electron and the charge of a neutron is equal to a bullet, then if you turn off the strong interaction, the interaction of the proton with electromagnetic field A will be the usual interaction of a Dirac particle - Yp / V For a neutron, electromagnetic interaction would be missing.
Designations: 67 - charge difference between electron and proton; q is the neutron charge; qg is the absolute value of the electron charge.
The nucleus consists of positively charged elementary particles - protons and not charge carriers neutrons.
The basis of modern ideas about the structure of matter is the assertion of the existence of atoms of matter, consisting of positively charged protons and chargeless neutrons, forming a positively charged nucleus, and negatively charged electrons rotating around the nucleus. The energy levels of electrons, according to this theory, are discrete in nature, and the loss or acquisition of some additional energy by them is considered as a transition from one allowed energy level another. In this case, the discrete nature of the electronic energy levels becomes the reason for the same discrete absorption or emission of energy by an electron during the transition from one energy level to another.
We assumed that the charge of an atom or molecule is completely determined by the scalar sum q Z (q Nqn, where Z is the number of electron-proton pairs, (q qp - qe is the difference in the charges of the electron and proton, N is the number of neutrons, and qn is the charge of the neutron.
The nuclear charge is determined only by the number of protons Z, and its mass number A coincides with the total number of protons and neutrons. Since the charge of the neutron is zero, electrical interaction according to Coulomb's law between two neutrons, as well as between a proton and a neutron is absent. At the same time, between two protons, electrical force repulsion.
Further, within the limits of measurement accuracy, not a single collision process has ever been registered, in which the charge conservation law would not be observed. For example, the inflexibility of neutrons in homogeneous electric fields allows us to consider the charge of the neutron as equal to zero with an accuracy of 1 (H7 of the electron charge.
We have already said that the difference between the magnetic moment of a proton and one nuclear magneton is an amazing result. Even more surprising (It seems that there is a magnetic moment for a neutron without a charge.
It is easy to see that these forces are not reduced to any of the types of forces considered in the previous parts of the physics course. Indeed, if we assume, for example, that gravitational forces act between nucleons in nuclei, then it is easy to calculate from the known masses of the proton and neutron that the binding energy per particle will be negligible - it will be 1036 times less than that observed experimentally. The assumption about the electric character also disappears. nuclear forces. Indeed, in this case it is impossible to imagine a stable nucleus consisting of a single charged proton and no charge of a neutron.
The strong bond that exists between nucleons in the nucleus indicates the presence in atomic nuclei of special, so-called nuclear forces. It is easy to see that these forces are not reduced to any of the types of forces considered in the previous parts of the physics course. Indeed, if we assume, for example, that gravitational forces act between nucleons in nuclei, then it is easy to calculate from the known masses of the proton and neutron that the binding energy per particle will be negligible - it will be 1038 times less than that observed experimentally. The assumption about the electric nature of nuclear forces also disappears. Indeed, in this case it is impossible to imagine a stable nucleus consisting of a single charged proton and no charge of a neutron.
NEUTRON. In 1930, German scientists W. Bothe and G. Becker were baffled by this phenomenon. By bombarding a plate of metallic beryllium with alpha particles, they discovered very weak, but surprisingly penetrating radiation emanating from the target, which even lead screens tens of centimeters thick, which blocked the most powerful gamma radiation, could not noticeably weaken.
The talented French physicists Frederic Jo-liot and Irene Curie noticed an even more curious fact. If a plate of paraffin, a substance rich in hydrogen, was placed in the path of this strange radiation, then protons, the nuclei of hydrogen atoms, began to fly out of the paraffin at great speed, and therefore with great energy.
Alpha particles were completely stuck in the beryllium plate and could not get into the paraffin. Knocking out protons with an energy of about 50 MeV from paraffin would be beyond the power of gamma rays. In this case, what kind of super-powerful "artillery" suddenly appeared in beryllium, and with what "shells" did it fire on paraffin?
Rutherford's student, the English physicist J. Chadwick, who had been studying mysterious radiation for a long time, finally came to the only possible and correct conclusion: having no electric charge, neither positive nor negative. These particles were later called neutrons.
Due to the absence of an electric charge, any substance becomes, as it were, “transparent” for the neutron. He calmly overcomes all the protective lines of the atom: and the outer electron shell, which repels any negatively charged particle with great force, and the total positive charge of the atomic nucleus, which throws aside even a heavy alpha particle moving at great speed.
The discovery of the neutron solved the mystery of the incomprehensible and "illogical" weight of the atomic nuclei with an increase in their positive charge by only one and allowed the Soviet scientist D. D. Ivanenko and the German scientist W. Heisenberg to propose in 1932 a new model of the structure of the atomic nucleus, in which everything turned out to be "simple and clear".
According to this model, the nuclei of all atoms consist of protons and neutrons. The number of protons is equal to the atomic number of the element in the periodic system of D. I. Mendeleev, and the masses of protons and neutrons put together are equal to its atomic mass, or mass number(see Nucleon). For example, the nucleus of a helium atom, known as the alpha particle, consists of two protons, which give it two positive electrical charges, and two neutrons. Total number protons and neutrons is four, which is exactly equal to its atomic weight, which for a long time caused bewilderment of scientists. Similarly, the nucleus of a lithium atom contains three protons (atomic number 3) and three neutrons, which add up to an atomic weight of six for the element.
The discovery of the neutron quite simply explains another mystery - the existence of isotopes. As an example, we can take the simplest chemical element in nature - hydrogen, the nucleus of which consists of a single proton. It is sometimes called protium. Then follows the heavy isotope of hydrogen, which has one proton and one neutron in the nucleus, with atomic mass equal to two. This isotope of hydrogen is called deuterium. Finally, there is a very rare, almost not found in nature, superheavy and already radioactive isotope hydrogen, in the nucleus of which there are two neutrons per proton. They called it tritium.
The new model of the structure of the atomic nucleus, in our description, perhaps even too simplified, almost completely explained the numerous facts accumulated by physics, and most importantly, opened up new ways for invading the holy of holies of the atom - its nucleus and, as was customary in science, treacherously failed to new, even deeper mysteries, contradictions and real miracles! To enumerate these peculiarities and marvels would be simply to recount from beginning to end the whole of modern nuclear physics. Therefore, here we will confine ourselves to a story about what is more or less directly and immediately connected with the neutron.
For example, why does the nucleus of an atom, which along with neutrons also includes positively charged protons, not fall apart under the action of truly titanic repulsive forces of like charges of protons (given the small distances between them)? It was only much later that it was established that special, unlike anything else, so-called intranuclear forces act within the nucleus, attracting these particles to each other, regardless of whether they are charged or neutral, and that these forces, acting on extremely small distances far exceed the repulsive forces of all protons taken together. Without these forces, nuclear particles would have scattered apart long ago, but rather, they would never have gathered together (see Nuclear Forces).
But in nature there are no and cannot exist any bodies, even the size of nuclear particles, which would not be in continuous motion, depending on temperature, i.e., the energy of the particles of matter composed of these particles. If an additional amount of energy enters this system of particles from somewhere outside, then the particles begin to move much faster. And, of course, a moment may come when this movement becomes so violent that one or even several particles will have the opportunity, having overcome the intranuclear forces, to jump out of their sphere of action. And then, already under the action of repulsive forces of like charges, this particle or several particles fly out of the nucleus of the atom.
If, on the other hand, much more excess energy arrives, all the particles of the nucleus of the atom, having pushed even more energetically, will be able to overcome the mysterious boundary of the action of intranuclear forces. Then the core will split on its own. How much of this excess energy, or excitation energy, as physicists call it, is needed in this case? The less, the heavier the nucleus of the atom. But on the other hand, the heavier the nucleus of an atom, the more energy is released during its “collapse”:
The heaviest nuclei are also the most unstable. And it is worth a little "push" them, that is, to give them a small amount of excess energy (in our example, 5 MeV), as the nucleus, saturated, like a sponge, with its own energy, will further divide by itself!
This can be done in two ways. The most difficult thing is to try to “drive” into the nucleus by force any heavy charged particle capable of overcoming the desperate resistance of the total positive electric charge of the atomic nucleus. But for this, the initial energy of 5 MeV is obviously not enough for a proton or an alpha particle. Most of its particles will be spent on overcoming the "armor protection" - the positive charge of the nucleus of an atom, for example, uranium-235, and, exhausted, they will not even be able to touch it, let alone separate it.
In addition, heavy particles, even with such energy, are not emitted by natural radioactive substances. Consequently, they need to be accelerated to much higher energies and speeds artificially on special installations - particle accelerators.
The neutron possesses quite different, truly amazing possibilities. Since the neutron does not carry an electric charge, it does not need any energy to overcome the total repulsive action of the positive charge of the atomic nucleus. Taking advantage of its neutrality, it freely penetrates to the nucleus of an atom, reaches the zone of attraction of intranuclear forces and is drawn into the nucleus. Having drawn in a neutron, the nucleus begins an internal restructuring. At the same time, it turns out to be the owner of an excess of energy equal to not 5, but 7 MeV, from which, naturally, having come into an excited state, it must immediately get rid of. Consequently, the mere simple addition of a neutron to the nucleus of a heavy uranium-235 atom introduces into it an additional energy equal to 7 MeV.
Where does this excess energy come from? Naturally, no miracles happen here. In the process of internal reconstruction of the old nucleus of an atom into a new one, the sum of the masses of all its nucleons turns out to be somewhat less than the sum of the masses of the nucleons taken separately. Due to this difference in masses, an equivalent amount of energy appears (see Mass Defect), first exciting the nucleus, and then leading it to fission. It turns out that for this the neutron should not have any initial energy at all. It is only necessary to help him get into the nucleus of the desired atom, and only there he, having mobilized the hidden reserves of the energy of the nucleus, will be able to release (though losing a little in mass) energy capable of blowing up the nucleus.
But neutrons that do not have any significant initial energy can divide the nuclei of not all elements, but only those in which the excitation energy necessary for their fission is less than 7 MeV, i.e., exactly the one that is released during the rearrangement of the nucleus, caused by adding an extra neutron to it. There are few such atoms: these are uranium-233, uranium-235, plutonium-239.
Here it is permissible to ask: where does the neutron come from such unusual, sharply different properties and abilities from other nuclear particles, although they also have their own rather amazing properties?
The origins of everything unusual lie in duality - the dualism of the properties of light, which behaves both as particles and as electromagnetic waves. Scientists were even more excited by the subsequent discovery of the same properties of the electron. These discoveries were perfectly explained by the theory put forward in 1900 by the German physicist Max Planck, according to which the radiation of heat or light by a body does not occur continuously, but discretely, i.e., in separate, strictly defined portions - quanta, and a light wave, having a very specific extent, in some cases exhibits properties characteristic of particles. In 1923, the French physicist Louis de Broglie established that specific wave properties inherent in any moving particle. According to his theory, the wavelength of any particle is directly proportional to some very small quantity called Planck's constant, and inversely proportional to the product of the particle's mass and its speed.
This ratio looks quite simple: X = himv. It follows from this relationship that the greater the mass or speed of a particle, or both at the same time, the shorter the wavelength inherent in it, and vice versa.
The laws of physics do not tolerate exceptions. And an object of the macrocosm, for example, a projectile or Earth, along with the properties of "particles" must also have wave properties. But due to their large mass, the wavelength corresponding to them is so small that these wave properties can be completely neglected. High-velocity neutrons have such a short wavelength that they actually behave like particles. Some features of their "strange" behavior can only be explained by explicitly wave properties. But since the mass of the neutron is still negligibly small in comparison with any, even microscopically small, body, the length of the nth wave becomes a quite perceptible quantity in the microcosm.
In order for the behavior of the neutron to show sufficient wave properties, its velocity must be as low as possible. It can be slowed down so much that the neutron completely loses the properties of the particle and behaves like a real wave.
Because of these features, obvious complications arise in establishing the true dimensions of the neutron, because, strange as it may seem, they depend on the velocity of this particle. For example, the diameter of an ordinary atom is approximately (2-4) 10-8 cm. The diameter of the nucleus is even smaller - about 2"10-13 cm. In order for the neutron wavelength to approximately correspond to the diameter of the atom, i.e. 10"8 cm, its energy (i.e., the speed of motion) should be only about 0.1 eV. A neutron with such a low energy is more correctly represented as a wave 10"8 cm long, and not as a particle of the same dimensions.
But then the paradoxes begin. A neutron with a wavelength of 10-8 cm turns out to be tens of thousands of times larger than the nucleus, which in turn contains neutrons, and not just one, but sometimes many!
A neutron can be inside the nucleus only if it moves with high speed J", therefore, has a short wavelength. And more speed, as we know, means more energy. Therefore, the neutrons that make up the nucleus have an energy of about 50 MeV, which corresponds to a very short wavelength - about 10-13 cm. This circumstance made it possible to explain the secret of the beta decay of radioactive substances, which has long tormented scientists and confused all their maps.
Having flown into the nucleus of an atom alien to him and creating an utter commotion there, the neutron cannot withstand the most complex interactions that have arisen, equivalent to monstrously high temperatures, and decays into a proton and an electron.
This discovery allowed scientists to consider the proton and neutron as one nuclear particle. Hence their name - nucleons. They can exist only in any one state: proton or neutron.
In beta decay, one of the neutrons becomes a proton. That's when the electron appears. Its charge must compensate for the positive charge of the newly born proton. However, due to the laws governing the radioactive decay of unstable nuclei, the electron does not have its place in the orbit, and it is forced to leave the nucleus. This will be the beta particle. The total positive charge of the still unstable nucleus becomes one more.
In turn, under certain conditions, a proton can turn into a neutron. But then somewhere it must disappear positive charge. This charge is carried away by the particle, which is an exact copy of the electron, but has the opposite, positive, charge. Such a particle was discovered in 1932. American physicist K. Anderson and named the positron. Both of these transformations are accompanied by the emission of another neutral particle - the neutrino.
The neutrons emitted by the beryllium source travel with great speed. Consequently, their effective size or, as they say, cross section is very small.
Colliding with the nuclei of atoms of light elements that meet on the way, neutrons bounce off them and change the direction of flight in much the same way as billiard balls bounce off each other. Each such collision costs the neutron some of its energy, so its speed slows down and its size, or cross section, increases.
Scientists took advantage of this to slow down its movement by repeated collisions of a neutron with substances containing atoms close in mass to a neutron (hydrogen, helium, carbon). Without being able to directly observe the neutron itself, it is easy to detect and measure the speed and energy of all the atoms “hit” and rebounding from it, and thereby the speed and energy of the neutron itself.
The neutron as a particle turned out to be slightly heavier than the proton. Outside the nucleus of an atom, it is radioactive and, having been free for about 11.7 minutes, it begins to decay: turning into a proton, it emits an electron and a neutrino. The amount of energy released during the decay of a neutron is approximately 1 MeV. This explains why the neutron is slightly heavier than the proton.
Observing the behavior of neutrons, scientists soon discovered another of their amazing features: easily penetrating through thick steel armor, they are not able to overcome even a thin plate of cadmium, which is easily penetrated not only by gamma radiation, but even by a stream of beta particles (electrons).
Soon this "strangeness" was also unraveled.
The nuclei of atoms of some elements (cadmium, boron, graphite, etc.) instead of repelling the neutron, "capture", draw it into themselves. The slower the neutron moves, the more successful such capture is.






