Features of condensation. What is condensation? Detailed analysis
Material from ThermalWiki - encyclopedia of heating
Condensation(Late Latin condensatio- condensation, from Latin condenso condense, thicken) - the transition of a substance from gaseous state into a liquid or solid due to its cooling or compression. Vapor condensation is possible only at temperatures below the critical temperature for a given substance. Condensation, as well as the reverse process - evaporation, is an example phase transformations substances ( phase transitions 1st kind). During condensation, the same amount of heat is released that was spent on the evaporation of the condensed substance. Rain, snow, dew, frost - all these natural phenomena are the result of the condensation of water vapor in the atmosphere.
So if you don't talk ugly about it, especially if your friends are fans of the Big Bang Theory, or if they are really real physicists, it is important to conclude the following. Condensed matter physics should be considered as a field of physics intended to be explored in a more thorough study of everything that encompasses physics in its solid state, as well as in its solid amorphous and liquid states.
Air is made up of several gases, among them. For clouds to form, the water vapor contained in a portion of the air must condense, that is, from a gaseous state to a liquid state. We are talking about condensation processes and other phase transformations.
Types of condensation
Two modes of surface condensation are known: film and drip. The first is observed during condensation on a wetted surface, it is characterized by the formation of a continuous film of condensate. On non-wetted surfaces, condensate forms as separate droplets. With drop condensation, the intensity of heat transfer is much higher than with film condensation, since a continuous condensate film makes heat transfer difficult.
So clouds are basically liquid water. Yes, these are water microdroplets! They are so small that we cannot see the shape of the fall. These microdroplets are all grouped together to form clouds. A pot of food or very hot water A: Many people say that "steam" or "smoke" comes out of the pot. There cannot be steam, because we have seen here that it is a gas, and therefore it is invisible. It also can't be smoke, as we don't have something on fire.
Figure 1: The elevated temperature of the pot caused the water to boil, changing from a liquid to a gaseous state. Water vapor on contact with air returns to liquid state, in a process called condensation, forming this little cloud just above the pan.
The rate of surface condensation is the higher, the lower the surface temperature compared to the saturation temperature of the vapor at a given pressure. The presence of another gas reduces the rate of surface condensation, since the gas makes it difficult for steam to reach the cooling surface. In the presence of non-condensable gases, condensation begins when the vapor at the cooling surface reaches the partial pressure and temperature corresponding to the saturation state (dew point).
From the bath and the entire bathroom "fog". The use of the word hazy is even correct because it comes from mist, a word that means cloud. We often mistakenly talk about smoke or steam. But then again: this is not steam, but a cloud! Figure 2: Part hot water coming out of the shower evaporates. And this vapor condenses into the environment.
In some places in Brazil we have low temperatures in certain time of the year. In the morning we left the house warm and warm. Then we give a "puff" and we see a mini-cloud come out of our mouths. Figure 3: A girl expels air from her mouth on a very cold day. The water vapor contained in this air condenses to find the lowest temperature outside.
Condensation can also occur inside the volume of steam (vapor-gas mixture). For bulk condensation to begin, the vapor must be appreciably supersaturated. The measure of supersaturation is the ratio
Details Category: Molecular-kinetic theory Posted on 09.11.2014 21:08 Views: 8235In a liquid state, a substance can exist in a certain temperature range. At a temperature below the lower value of this interval, the liquid turns into a solid. And if the temperature value exceeds the upper limit of the interval, the liquid passes into a gaseous state.
Why do the above phenomena occur? Why did water vapor find the conditions for the transition to a liquid state. Figure 4: Water vapor condenses on a frozen channel! In the case of clouds, whether artificial, as listed above, or natural, a surface with a cooler temperature is also required. Think of the hot and humid day that is commonly experienced in the summer of most of Brazil. On such a day, the surface of our planet heats up rapidly. Think about it when it's hard to walk barefoot on the beach or on pavement that day.
Figure 5: In some situations, the surface and the thin layer of air above it is much warmer than the rest of the air, forming a mirage phenomenon. Our planet's surface then heats up the air above it in the first few meters of the atmosphere. So these portions of warm air are forced to rise as warmer air is less dense. To understand this, let's remember, for example, balloons. Balloons heat the air inside the balloon, and this air, being lighter, is forced to rise.
We can observe all this in the example of water. In a liquid state, we see it in rivers, lakes, seas, oceans, a water tap. Solid state water is ice. It turns into it when, at normal atmospheric pressure, its temperature drops to 0 o C. And when the temperature rises to 100 o C, water boils and turns into steam, which is its gaseous state.
Figure 6: Hot gas balloons: The air inside the balloon is heated by a gas flame. Because the air inside the balloon is hotter than the surrounding air, it is therefore less dense and therefore rises. Each piece of hot air above the surface then behaves like an "invisible ball".
Figure 7: Some birds, such as the condor, buzzard, and kingfisher, use rising hot air to simply glide, that is, fly without flapping their wings. Some birds use these patches of hot air to glide and remain calm while flying without having to close their wings. The same procedure is used by hang gliders, ultralights and paragliders.
The process of changing a substance into vapor is called vaporization. The reverse process of changing from vapor to liquid is condensation .
Vaporization occurs in two cases: during evaporation and during boiling.
Evaporation
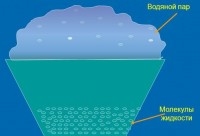
Figure 8: Ultralights, paragliders and hang gliders are designed to use resorts to climb and make their movements. If these areas rise to the point where they encounter cooler temperatures that favor condensation, the water vapor contained in these areas can condense and then form clouds.
Figure 9: The process of cloud formation through hot springs, which are parts of hot air that rise because they are less dense. Recall the case of tin. The surrounding air found the colder surface of the jar, and then the water vapor contained in the air condensed. But in the case of an air parcel that formed a cloud? On what surface did water vapor condense to form these droplets?
Evaporation is the phase process of the transition of a substance from a liquid state to a gaseous or vaporous state, occurring on the surface of the liquid .
As with melting, heat is absorbed by a substance during evaporation. It is spent on overcoming the cohesive forces of particles (molecules or atoms) of the liquid. Kinetic energy molecules with the highest speed exceeds their potential energy interactions with other liquid molecules. Due to this, they overcome the attraction of neighboring particles and fly out from the surface of the liquid. The average energy of the remaining particles becomes smaller, and the liquid gradually cools down if it is not heated from the outside.
In the atmosphere, there are tiny particles in suspension, invisible to the naked eye. These particles are called condensation nuclei because they have an affinity for water. This is the surface on which water vapor condenses. Condensation nuclei can be pollen, dust, salt particles, smoke, etc.
Figure 10: Many particles can act as condensation nuclei. From natural to man-made particles. In the figure we have a huge cloud of pollutants in India. When water condenses on these particles, then we have very small droplets that form a single cloud!
Since particles are in motion at any temperature, evaporation also occurs. at any temperature. We know that puddles dry up after rain, even in cold weather.
But the rate of evaporation depends on many factors. One of the most important - substance temperature. The higher it is, the greater the speed of particles and their energy, and the greater their number leaves the liquid per unit time.
In future posts we will have more information on this issue. Some of the photographs that are illustrated were taken from the stock service. For getting additional information click on the photo. In the atmosphere we find all phenomena related to climate. However, some of them occur very close to the Earth's surface and directly depend on it.
Below we will discuss some of these phenomena related to the physical state of water change and, strictly, the surface of the Earth. These are the so-called forms of condensation: dew, frost, fog and clouds. At night, calm and clear sky Earth's surface releases large amounts of energy into space, cooling rapidly in a process known as radiative nighttime cooling. The layer of air lying on top of the surface soon loses heat.
Fill 2 glasses with the same amount of water. We put one in the sun, and the other we leave in the shade. After a while, we will see that there is less water in the first glass than in the second. It was heated by the sun's rays, and it evaporated faster. Puddles after rain also dry up much faster in summer than in spring or autumn. In extreme heat, rapid evaporation of water from the surfaces of reservoirs occurs. Ponds and lakes are drying up, the beds of shallow rivers are drying up. The higher the temperature environment, the higher the evaporation rate.
The loss of heat from this layer of air results in the loss of the capacity of this air to store water vapor. Therefore, we say that the saturation with saturated air of saturation and the excess amount of water present in this layer passes into a liquid state, that is, condenses. Then the water particles settle on the surface in the form of droplets.
Frost has a formation process very similar to dew. Under these conditions, the resulting dew can solidify, forming ice crystals or sublimating water vapor passing right through the solid state. White Frost: This is the most typical. It does this by depositing ice crystals on the surface of objects. This happens in humid air and usually doesn't cause as much trouble.
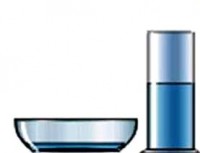
With the same volume, the liquid in a wide plate will evaporate much faster than the liquid poured into a glass. It means that evaporation rate depends on the surface area of evaporation . The larger this area, the greater the number of molecules flies out of the liquid per unit time.
Occurs when plant tissues are frozen without ice deposition. It happens in dry air and does a lot of damage. agriculture and even crop losses. It also brings great damage to agriculture. Fog is a suspension of fine particles of liquid or icy water, which can result in a significant reduction in visibility.
As a convention, the term "fog" is used when the horizontal visibility is less than 1 km. When this visibility is greater than 1 km, we call this phenomenon fog or mist. Various reasons can be associated with the formation of fog. The most common type occurs on calm and damp nights where, like frost and dew, the surface of objects cools rapidly. Surface layer Air that is humid and requires only a slight drop in temperature to reach its dew point saturates to form small droplets of water and ice that are not heavy enough to settle and suspend.
With the same external conditions evaporation rate depends on the type of substance . Fill the glass flasks with the same volume of water and alcohol. After a while, we will see that there is less alcohol left than water. It evaporates at a faster rate. This happens because alcohol molecules interact more weakly with each other than water molecules.
This type of fog is called radiation fog and is common in valleys. Another type of general fog is orographic fog. Humid air that prevents lower areas, when it finds higher ground like a hill, rises, expands and cools. As it cools, the mass of air reaches the dew point, forming fog.
However, there are two other types of fog: advection and steam. The first of these is formed when a layer of hot and humid air comes into contact with a cold surface if it is cooling. The second occurs in bodies of water when cold air comes into contact with hot water, causing it to evaporate and consequently increase moisture in the air mass. Thus, this mass reaches saturation.
affect the rate of evaporation and presence of wind . We know that things after washing dry much faster when they are blown by the wind. The jet of hot air in a hair dryer can quickly dry our hair.
The wind carries away the molecules that have flown out of the liquid, and they do not return back. Their place is taken by new molecules leaving the liquid. Therefore, they become less in the liquid itself. Therefore, it evaporates faster.
Clouds and fog, roughly speaking, the same thing. The difference, however, is that the clouds are located at a much higher altitude, while the fog forms at ground level. The main process of cloud formation occurs by adiabatic expansion. In this process, a change in volume and temperature is observed without heat transfer. A mass of hot air near the surface rises and expands under less pressure. By increasing the space between the particles of this mass, the temperature drops, which leads to saturation.
Evaporation and condensation are two processes in which matter changes from one state to another. Matter exists in three different states: solid, liquid and gaseous. When evaporated, the liquid material becomes gaseous. When condensed, a gaseous substance becomes a liquid.
Sublimation

Evaporation takes place in solids Oh. We see how the frozen, ice-covered linen gradually dries out in the cold. Ice turns into steam. We smell the pungent odor produced by evaporation solid matter naphthalene.
All matter is made up of tiny moving particles called molecules. Evaporation and condensation occur when these molecules gain or lose energy in the form of heat. As the sun warms the water in the puddle, it slowly subsides. Similarly, when water boils in a saucepan, its level decreases. These are two examples of evaporation. The water appears to disappear, but actually passes into the air as a gas called water vapour.
Evaporation occurs when the liquid is heated. Heat gives more energy to the molecules of the liquid. This energy makes the molecules move faster. Given sufficient energy, molecules near the surface are released from the liquid and enter the air as a gas.
Some substances do not have a liquid phase at all. For example, elemental iodineI 2 - a simple substance, which is black-gray crystals with a purple metallic luster, under normal conditions immediately turns into gaseous iodine - purple vapor with a pungent odor. The liquid iodine that we buy in pharmacies is not its liquid state, but a solution of iodine in alcohol.
The transition process of solids into a gaseous state, bypassing the liquid stage, is called sublimation, or sublimation .
Boiling

Boiling This is also the process of liquid turning into vapor. But vaporization during boiling occurs not only on the surface of the liquid, but throughout its entire volume. Moreover, this process is much more intense than during evaporation.
Put a kettle of water on the fire. Since there is always air dissolved in water, when heated, bubbles appear on the bottom of the kettle and on its walls. These bubbles contain air and saturated water vapor. First they appear on the walls of the teapot. The amount of steam in them increases, and they themselves increase in size. Then, under the influence of the buoyant force of Archimedes, they will break away from the walls, rise up and burst on the surface of the water. When the water temperature reaches 100 ° C, bubbles will form throughout the entire volume of water.
Evaporation occurs at any temperature, and boiling occurs only at a certain temperature, which is called boiling point .
Each substance has its own boiling point. It depends on the amount of pressure.
At normal atmospheric pressure, water boils at a temperature of 100 o C, alcohol - at 78 o C, iron - at 2750 o C. And the boiling point of oxygen is minus 183 o C.
As the pressure decreases, the boiling point decreases. In the mountains where Atmosphere pressure lower, water boils at a temperature of less than 100 o C. And the higher above sea level, the lower the boiling point. And in a pressure cooker, where increased pressure is created, water boils at a temperature above 100 o C.
Saturated and unsaturated steam
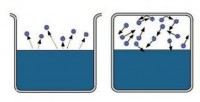
If a substance can simultaneously exist in a liquid (or solid) phase and a gaseous one, then its gaseous state is called ferry . Vapor is made up of molecules escaping from a liquid or solid during evaporation.
Pour the liquid into the vessel and close it tightly with a lid. After a while, the amount of liquid will decrease due to its evaporation. Molecules leaving the liquid will concentrate above its surface in the form of vapor. But when the vapor density becomes quite high, some of them will begin to return to the liquid again. And there will be more and more such molecules. Finally, a moment will come when the number of molecules leaving the liquid and the number of molecules returning to it will be equal. In this case, they say that liquid is in dynamic equilibrium with its vapor . This pair is called rich .
If, during vaporization, more molecules fly out of the liquid than return, then such vapor will be unsaturated . unsaturated steam formed when the evaporating liquid is in an open vessel. The molecules leaving it are scattered in space. Not all of them return to the liquid.
Steam condensation

The reverse transition of a substance from a gaseous state to a liquid state is called condensation. During condensation, some of the vapor molecules return to the liquid.
Steam begins to turn into a liquid (condense) at a certain combination of temperature and pressure. This combination is called critical point . Maximum temperature , below which condensation begins is called critical temperature. Above the critical temperature, the gas will never turn into a liquid.
AT critical point the liquid-vapor interface is blurred. disappears surface tension liquid, the densities of the liquid and its saturated vapor are equalized.
At dynamic equilibrium, when the number of molecules leaving the liquid and returning to it is equal, the processes of evaporation and condensation are balanced.
When water evaporates, its molecules form water vapor , which is mixed with air or other gas. The temperature at which such vapor in the air becomes saturated, begins to condense upon cooling and turns into water droplets, is called dew point .

When there is a large amount of water vapor in the air, it is said that its humidity is increased.
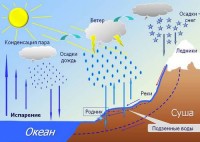
We observe evaporation and condensation very often in nature. Morning fog, clouds, rain - all this is the result of these phenomena. FROM earth's surface moisture evaporates when heated. The molecules of the resulting vapor rise up. Encountering cool leaves or blades of grass on its way, the steam condenses on them in the form of dew drops. A little higher, in the surface layers, it becomes fog. And high in the atmosphere at low temperatures, the cooled vapor turns into clouds consisting of water droplets or ice crystals. Subsequently, rain or hail will fall from these clouds to the earth.
But water droplets form during condensation only when the smallest solid or liquid particles are in the air, which are called condensation nuclei . They can be products of combustion, spraying, dust particles, sea salt over the ocean, particles formed as a result of chemical reactions in the atmosphere, etc.
desublimation

Sometimes a substance can go from a gaseous state immediately to a solid state, bypassing the liquid stage. Such a process is called desublimation .
Ice patterns that appear on glasses in cold weather are an example of desublimation. During frosts, the soil is covered with hoarfrost - thin ice crystals into which water vapor has turned from the air.