Incident photon energy formula. Photon theory of light. Mass, energy and momentum of a photon
AT modern interpretation the quantum hypothesis states that the energy E vibrations of an atom or molecule can be equal to h v, 2 h v, 3 hν, etc., but there are no oscillations with energy between two successive integer multiples of . This means that energy is not continuous, as has been believed for centuries, but quantized , i.e. exists only in strictly defined discrete portions. The smallest portion is called energy quantum . The quantum hypothesis can also be formulated as a statement that vibrations at the atomic-molecular level do not occur with any amplitudes. Permissible amplitude values are related to the oscillation frequency ν .
In 1905, Einstein put forward a bold idea that generalized the quantum hypothesis and put it at the basis of a new theory of light (the quantum theory of the photoelectric effect). According to Einstein's theory , light with frequencyν Not only emitted, as Planck suggested, but also propagates and is absorbed by matter in separate portions (quanta), whose energy. Thus, the propagation of light should be considered not as a continuous wave process, but as a stream of discrete light quanta localized in space, moving at the speed of light propagation in vacuum ( With). Quantum electromagnetic radiation was named photon .
As we have already said, the emission of electrons from the surface of a metal under the action of radiation incident on it corresponds to the concept of light as an electromagnetic wave, since the electric field of the electromagnetic wave acts on the electrons in the metal and pulls out some of them. But Einstein drew attention to the fact that the details of the photoelectric effect predicted by the wave theory and the photon (quantum corpuscular) theory of light diverge significantly.
So, we can measure the energy of the emitted electron, based on the wave and photon theory. To answer the question of which theory is preferable, let's look at some details of the photoelectric effect.
Let's start with wave theory, and suppose that plate illuminated monochromatic light . The light wave is characterized by the parameters: intensity and frequency(or wavelength). Wave theory predicts that when these characteristics change, the following phenomena occur:
With increasing light intensity, the number of ejected electrons and their maximum energy should increase, because higher light intensity means greater amplitude electric field, and a stronger electric field pulls out electrons with more energy;
ejected electrons; the kinetic energy depends only on the intensity of the incident light.
Quite different is predicted by the photon (corpuscular) theory. First of all, we note that in a monochromatic beam all photons have the same energy (equal to h v). An increase in the intensity of a light beam means an increase in the number of photons in the beam, but does not affect their energy if the frequency remains unchanged. According to Einstein's theory, an electron is ejected from the surface of a metal when a single photon collides with it. In this case, all the energy of the photon is transferred to the electron, and the photon ceases to exist. Because electrons are held in the metal by attractive forces, the minimum energy is required to knock out an electron from the surface of the metal A(which is called the work function and is, for most metals, a value of the order of several electron volts). If the frequency ν of the incident light is small, then the energy and energy of the photon is not enough to knock out an electron from the surface of the metal. If , then the electrons fly out from the surface of the metal, and energy in this process is preserved, i.e. photon energy ( hν) is kinetic energy of the ejected electron plus the work of knocking the electron out of the metal:
| (2.3.1) |
Equation (2.3.1) is called Einstein's equation for the external photoelectric effect.
Based on these considerations, the photon (corpuscular) theory of light predicts the following.
1. An increase in light intensity means an increase in the number of incident photons, which knock out more electrons from the surface of the metal. But since the energy of the photons is the same, the maximum kinetic energy of the electron will not change ( confirmed I photoelectric law).
2. With an increase in the frequency of the incident light, the maximum kinetic energy of the electrons increases linearly in accordance with the Einstein formula (2.3.1). ( Confirmation II photoelectric effect law). The graph of this dependence is shown in Fig. 2.3.
 , ,
|
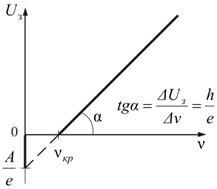
Rice. 2.3
3. If the frequency ν is less than the critical frequency , then there is no ejection of electrons from the surface (III law).
So, we see that the predictions of the corpuscular (photon) theory are very different from the predictions of the wave theory, but they agree very well with the three experimental established laws photoelectric effect.
Einstein's equation was confirmed by Millikan's experiments carried out in 1913–1914. The main difference from Stoletov's experiment is that the metal surface was cleaned in a vacuum. The dependence of the maximum kinetic energy on frequency was studied and Planck's constant was determined h.
In 1926, Russian physicists P.I. Lukirsky and S.S. Prilezhaev used the method of a vacuum spherical capacitor to study the photoelectric effect. The anode was the silver-plated walls of a glass spherical container, and the cathode was a ball ( R≈ 1.5 cm) from the investigated metal placed in the center of the sphere. This shape of the electrodes made it possible to increase the slope of the CVC and thereby more accurately determine the retarding voltage (and, consequently, h). The value of Planck's constant h obtained from these experiments agrees with the values found by other methods (by black-body radiation and by the short-wavelength boundary of the continuous x-ray spectrum). All this is proof of the correctness of Einstein's equation, and at the same time of his quantum theory of the photoelectric effect.
For explanation thermal radiation Planck suggested that light is emitted in quanta. Einstein, when explaining the photoelectric effect, suggested that light is absorbed by quanta. Einstein also suggested that light propagates in quanta, i.e. portions. The quantum of light energy is called photon . Those. again came to the concept of a corpuscle (particle).
The most direct confirmation of Einstein's hypothesis came from Bothe's experiment, which used the coincidence method (Fig. 2.4).
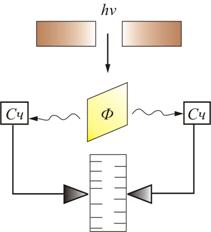
Rice. 2.4
Thin metal foil F placed between two gas-discharge counters mid. The foil was illuminated with a weak beam x-rays, under the influence of which she herself became a source of x-rays (this phenomenon is called x-ray fluorescence). Due to the low intensity of the primary beam, the number of quanta emitted by the foil was small. When quanta hit the counter, the mechanism worked and a mark was made on the moving paper tape. If the radiated energy were distributed uniformly in all directions, as follows from wave representations, both counters should have worked simultaneously and the marks on the tape would have fallen one against the other. In fact, there was a completely random arrangement of marks. This can only be explained by the fact that in separate acts of emission, light particles arise, flying first in one direction, then in the other. So the existence of special light particles - photons was experimentally proved.
Photon has energy
![]() . For visible light wavelength λ = 0.5 µm and energy E= 2.2 eV, for x-rays λ = μm and E= 0.5 eV.
. For visible light wavelength λ = 0.5 µm and energy E= 2.2 eV, for x-rays λ = μm and E= 0.5 eV.
A photon has an inertial mass , which can be found from the relation:
| ; |
| (2.3.2) |
A photon moves at the speed of light c\u003d 3 10 8 m / s. Let's substitute this speed value into the expression for the relativistic mass:
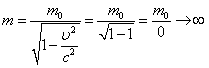 . .
|
A photon is a particle that has no rest mass. It can only exist by moving at the speed of light c .
Let us find the relationship between energy and momentum of a photon.
We know the relativistic expression for momentum:
| . | (2.3.3) |
And for energy:
| . | (2.3.4) |
According to Einstein's hypothesis of light quanta, light is emitted, absorbed and propagated in discrete portions (quanta) calledphotons.
Photon energy E = h .
Weight movements photon m γ is found from the law of the relationship of mass and energy
A photon is an elementary particle that always moves at the speed of light. With and has a rest mass of zero. Consequently, the mass of a photon differs from the mass of such elementary particles as the electron, proton and neutron, which have a non-zero rest mass and can be at rest.
photon momentum R γ is determined by the formula
 . (1.20)
. (1.20)
So, as we see, the photon, like any other particle, is characterized by energy, weight and momentum.
If photons have momentum, then the light falling on the body must have an effect on it. pressure. From the point of view of quantum theory, the pressure of light on the surface is due to the fact that each photon, when colliding with the surface, transfers its momentum to it.
Light pressure is determined by the formula
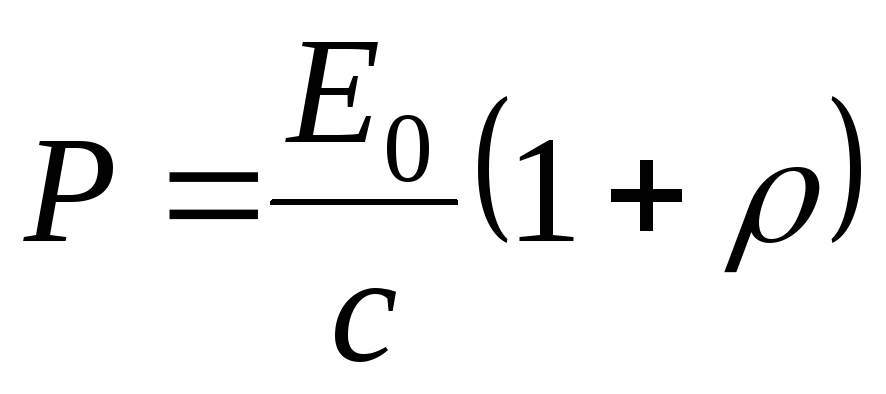 , (1.21)
, (1.21)
where 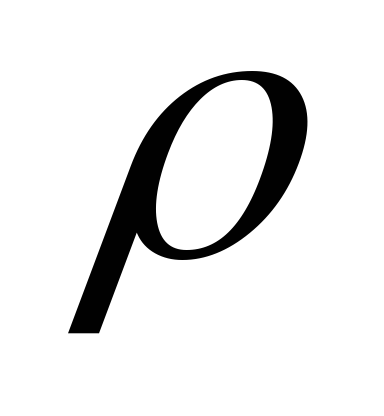 is the light reflection coefficient;
is the light reflection coefficient;  E 0
is the energy incident on a unit surface per unit time (radiation power E 0
=
Nhv,
where
N is the number of photons incident on a unit surface per second).
E 0
is the energy incident on a unit surface per unit time (radiation power E 0
=
Nhv,
where
N is the number of photons incident on a unit surface per second).
§1.3 The dual nature of the electromagnetic radiation of matter
Compton effect
Compton effect called elastic scattering of short-wave electromagnetic radiation (X-ray and γ - radiation) on free (or weakly bound) electrons of a substance, accompanied by an increase in wavelength.
The explanation of the Compton effect is given on the basis of quantum concepts of the nature of light. If we assume that the radiation is of a corpuscular nature, i.e. represents a stream of photons, then the Compton effect is the result of an elastic collision of X-ray photons with free electrons of matter (for light atoms, electrons are weakly bound to the nuclei of atoms, so they can be considered free). During this collision, the photon transfers to the electron part of its energy and momentum in accordance with the laws of their conservation.
Experimentally, Compton obtained the following expression
where λ
1
is the wavelength of the scattered quantum; λ
is the wavelength of the incident quantum; λ
to
=2,43∙10
-12
m - Compton wavelength(when a photon is scattered by an electron); m 0
is the rest mass of the electron;  is the scattering angle.
is the scattering angle.
If an electron is strongly bound to an atom, then when a photon is scattered by it, the latter transfers energy and momentum not to the electron, but to the atom as a whole. The mass of an atom is many times greater than the mass of an electron. Therefore, only a small part of the photon energy is transferred to the atom, so that the wavelength λ 1 scattered radiation practically does not differ from the wavelength λ incident radiation. The fraction of electrons that are strongly bound in atoms increases with the mass of the atoms. Therefore, the heavier the atoms of the scattering material, the greater the relative intensity of the unshifted component ( λ 1 =λ ) in scattered radiation.
In contrast to photon scattering, which occurs both on free and bound electrons, photons can only be absorbed by related electrons. For example, with an external photoelectric effect, a photon is absorbed by a bound electron, which spends part of the energy received to perform the work function, which is a measure of the binding of an electron in a substance.
Absorption of a photon by a free electron is impossible, since this process would contradict the laws of conservation of energy and momentum.
According to Einstein's hypothesis of light quanta, light is emitted, absorbed and propagated in discrete portions (quanta) called photons. Photon energy ξ 0 =hv. Its mass is found from the law of the relationship between mass and energy:
Photon- elementary particle, which always (in any medium!) moves at the speed of light With and has a rest mass of zero. Consequently, the mass of a photon differs from the mass of such elementary particles as the electron, proton and neutron, which have a non-zero rest mass and can be at rest.
photon momentum pv we get if general formula theory of relativity IM rest mass of a photon m 0γ = 0:
![]() (4.2)
(4.2)
From the above reasoning it follows that a photon, like any other particle, is characterized by energy, mass and momentum. Expressions (205.1), (205.2) and (200.2) connect corpuscular characteristics of a photon - mass, momentum and energy of a wave th characteristic of light - its frequency v.
If photons have momentum, then light falling on a body must create pressure on it. According to quantum theory, the pressure of light on the surface is due to the fact that each photon, when colliding with the surface, transfers its momentum to it.
Let us calculate, from the point of view of quantum theory, the light pressure exerted on the surface of a body by a flux of monochromatic radiation (frequency v) incident perpendicular to the surface. If per unit time per unit area of the body surface falls N photons, then at the reflection coefficient R light from the surface of the body pN photons will be reflected, and (1-p) N- be absorbed. Each absorbed photon imparts momentum to the surface p Y =hv/c, and each reflected - 2p y =2hv/c(when reflected, the momentum of a photon changes by - RU). The pressure of light on the surface is equal to the momentum that the surface transmits in 1 s N photons:
Nhv = Ee is the energy of all photons incident on a unit surface per unit time, i.e., the energy illumination of the surface, a e/c=w volume density of radiation energy. Therefore, the pressure produced by light during normal incidence on the surface,
![]() (4.3)
(4.3)
Formula (4.3), derived on the basis of quantum concepts, coincides with the expression obtained from Maxwell's electromagnetic (wave) theory. Thus, the pressure of light is equally successfully explained by both wave and quantum theory. As already mentioned, experimental proof of the existence of light pressure on solid bodies and gases are given in the experiments of P. N. Lebedev, who at one time played an important role in the approval of Maxwell's theory. Lebedev used a light suspension on a thin thread, along the edges of which light wings are attached, some of which are blackened, while the surfaces of others are mirrored. To eliminate convection and the radiometric effect, a movable system of mirrors was used to direct light onto both surfaces of the wings; the suspension was placed in an evacuated balloon; The light pressure on the wings was determined from the angle of twist of the suspension thread and coincided with the theoretically calculated one. In particular, it turned out that the pressure of light on a mirror surface is twice that on a blackened one (see (4.3)).
Photon is an elementary particle, a quantum of electromagnetic radiation.
Photon energy: ε = hv, where h = 6.626 10 -34 J s is Planck's constant.
Photon mass: m = h·v/c 2 . This formula is obtained from the formulas
ε = hv and ε = m c 2 . The mass, defined by the formula m = h·v/c 2 , is the mass of the moving photon. A photon has no rest mass (m 0 = 0), since it cannot exist at rest.
Photon momentum: All photons move at a speed c = 3·10 8 m/s. Obviously, the momentum of the photon is P = m c, which implies that
P = hv/c = h/λ.
4. External photoelectric effect. Volt-ampere characteristic of the photoelectric effect. Stoletov's laws. Einstein's equation
The external photoelectric effect is the phenomenon of the emission of electrons by a substance under the action of light.
The dependence of the current on the voltage in the circuit is called the current-voltage characteristic of the photocell.
1) The number of photoelectrons N' e escaping from the cathode per unit time is proportional to the intensity of light falling on the cathode (Stoletov's law). Or in other words: the saturation current is proportional to the power of the radiation incident on the cathode: Ń f = P/ε f.
2) The maximum speed V max that an electron has at the exit from the cathode depends only on the frequency of light ν and does not depend on its intensity.
3) For each substance there is a limiting frequency of light ν 0, below which the photoelectric effect is not observed: v 0 = A out / h. Einstein's equation: ε = A out + mv 2 max /2, where ε = hv is the energy of the absorbed photon, A out is the work function of the electron from the substance, mv 2 max / 2 is the maximum kinetic energy of the emitted electron.
Einstein's equation, in fact, is one of the forms of writing the law of conservation of energy. The current in the photocell will stop if all the emitted photoelectrons slow down before reaching the anode. To do this, it is necessary to apply a reverse (delay) voltage u to the photocell, the value of which is also found from the law of conservation of energy:
|e|u s = mv 2 max /2.
5. Light pressure
Light pressure is the pressure exerted by light falling on the surface of a body.
If we consider light as a stream of photons, then, according to the principles of classical mechanics, when particles hit a body, they must transfer momentum, in other words, exert pressure. This pressure is sometimes called radiation pressure. To calculate the light pressure, you can use the following formula:
p = W/c(1+ p), where W is the amount of radiant energy incident normally on 1 m 2 of the surface in 1 s; c is the speed of light, p- reflection coefficient.
If the light falls at an angle to the normal, then the pressure can be expressed by the formula:
6. Compton - effect and its explanation
The Compton effect (Compton effect) is the phenomenon of changing the wavelength of electromagnetic radiation due to its scattering by electrons.
For scattering by an electron at rest, the frequency of the scattered photon is:

where is the scattering angle (the angle between the directions of photon propagation before and after scattering).
The Compton wavelength is a length dimension parameter characteristic of relativistic quantum processes.
λ C \u003d h / m 0 e c \u003d 2.4 ∙ 10 -12 m - the Compton wavelength of the electron.
An explanation of the Compton effect is impossible within the framework of classical electrodynamics. From the point of view of classical physics, an electromagnetic wave is a continuous object and should not change its wavelength as a result of scattering by free electrons. The Compton effect is a direct proof of the quantization of an electromagnetic wave, in other words confirms the existence of a photon. The Compton effect is another proof of the validity of the corpuscular-wave dualism of microparticles.






