What does g mole mean. A mole is a unit of quantity of a substance. Molar mass
Chemistry lesson in grade 8 on the topic “Amount of substance. Moth"
Lesson Objectives:
- to acquaint students with a new physical quantity - the amount of a substance, with its unit of measurement - a mole; learn to use the amount of matter and the mole.
Give the concept of molar mass, continue the formation of the concept of the amount of substance, the ability to use this value, enrich the content chemical formula.
Tasks:
To form during the lesson the concepts of "amount of substance", "Avogadro's number".
To form the ability to solve problems using the concepts of "amount of substance", "Avogadro number".
Continue to develop experimental skills in working with substances, the ability to observe, analyze, draw conclusions, highlight the necessary information.
Planned result:
Students know the concepts of "amount of substance", "Avogadro number", they can solve calculation problems using these concepts
Lesson type: combined ICT - a lesson using interactive teaching methods.
Lesson plan.
I stage. Organizing time
Greeting, creating a positive emotional mood.
Checking students for class.
Good afternoon guys!(mark missing)
I'm glad to see you. I see smart, kind faces in front of me. In order to understand how we will work today, I want to know what mood you are in. If you're in a good mood, smile at me. Look at each other, smile!
I am sure that today's lesson will bring us satisfaction and be fruitful, and your mood will not worsen by the end of the lesson.
II stage. Updating the necessary knowledge and skills, including verification homework(slide 2)
Where in the Periodic Table are the elements corresponding to simple substances metals?
Where are non-metals found in the Periodic Table?
What are the general physical properties metals?
What is allotropy?
Give examples of allotropy?
Complete the sentences: (slide 3)
Known 22 non-metal
Iodine crystals purple colors
liquid non-metal bromine
Carbon forms diamond and graphite
After a thunderstorm in the air is formed ozone
From the sentences below, write out the numbers corresponding to metals in one column, and the numbers corresponding to non-metals in another column. (slide 4)
1. Aggregate state: solid.
2. Have a metallic sheen.
3.Do not have a metallic sheen.
5. Electrically conductive.
6. Gaseous.
7. In the solid state - brittle.
8.Plastic.
9.Do not conduct heat.
Metals
1,2,4,5,8
non-metals
1,3, 6,7, 9
(slide 5) Assess your condition before a new topic.
III stage. Explanation of new material. (slide 6)
The glue spilled - the words stuck together. Separate the words from each other with dashes.
sugar|mole|water|quantity|one|substance|
The teacher communicates the goals and objectives of the lesson.
Recording the topic of the lesson (slide 7)
The teacher reminds students that chemistry is the science of substances and their transformations. To obtain a certain amount of product (in chemical laboratory or at the factory) it is necessary to take strictly defined quantities initial substances.
Chemists, conducting experiments, noticed that the composition of the products of some reactions depends on the ratios in which the reactants were taken.
(slide 8) For example, if coal is burned in excess of oxygen, carbon dioxide is produced:
C + O 2 →CO 2
And if you take 2 times less, you get carbon monoxide:
C + 0.5O 2 → CO 2
In the first case, 1 oxygen molecule reacted with 1 carbon atom, and in the second case, one oxygen molecule reacted with two carbon atoms.
The question arises: how to measure a certain number of atoms or molecules?
It is easy to measure the mass of a substance (with the help of scales) or the volume of a gas or liquid (with the help of measuring utensils). So you need physical quantity, which allows you to convert the number of atoms or molecules into mass or volume.
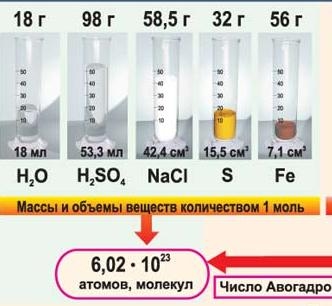
Let's carry out simple calculations: the mass of the lightest atom - hydrogen is 1.66 10 -24 g, this mass is taken as a unit of relative atomic mass: Ar(H) = 1. Now suppose we have 1 g of hydrogen atoms. How many atoms will there be in this mass? To calculate, you need to divide the mass of a portion of hydrogen by the mass of 1 hydrogen atom: 1 g: 1.66 11 -24 g ≈ 6.02 10 23 atoms.
In the mass (in grams) of any element, numerically equal to the relative atomic mass, will contain the same number of atoms of that element.
The mass (in grams) of any substance, numerically equal to its relative molecular weight, will contain the same number of molecules. This is a constant value, it is called Avogadro's number, denoted by N A .
(slide 9)
(slide 10)What associations does the drawing evoke? (mol). The unit for measuring the amount of a substance was called " mole"
The amount of a substance containing 6.02 10 23 structural units of a substance is called molem .(slide 11)
Based on the examples considered, the teacher adds that the number of other particles is also measured in moles: atoms, ions, electrons, etc.
(slide 12/1)1 mole of any substance in any state of aggregation contains N A = 6∙10 23 its molecules.
mole - unit amount of substance .
The amount of a substance is a physical quantity introduced to link the number of structural units of a substance and its mass or volume.
(slide 12/2) The amount of a substance is denoted n(en) or (nu) (obsolete), measured in moles.
n[mol]
(slide 13)General slide of the amount of substance.
(slide 14-15) 1 mol of any substance has a mass numerically equal to the relative molecular mass, but having a unit of g / mol
This mass is called molar , denoted by M.
Mr = M [g/mol]
How can you see 1 mole of a substance?
Take a tablespoon, fill it with water and pour it into a beaker. This will be approximately 1 mole of water. Pour another spoonful of water into a glass. Now there are 2 moles of this substance in the glass. Let's add one more spoon. The amount of water will become equal to 3 mol.
(slide 16)Demonstrate to the class some substances the amount of 1 mol: 56 g of iron powder ( Fe ), 58 g salt ( NaCl ), 342 g sugar ( C 12 H 22 O 11 )
(slide 17-18)Determination of the molar mass of certain substances
(slide 19) The amount of substance and mass are related by the following relationship:
m = n M
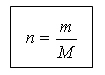
The amount of substance and the number of structural units N are related by the following relation:
N = N A n
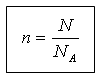
- So, how do we calculate the number of particles contained in three moles of water? (slide 20)
It is necessary to multiply the amount of the substance taken (in this case, water) by the Avogadro constant:
N(H 2 O) \u003d 3 mol 6 10 23 molecules \u003d 18 10 23
(slide 22) Assess your condition in the lesson
IV stage. Consolidation. Problem solving.
Find the mass of 10 moles of nitrogen? (slide 23)
Solution:
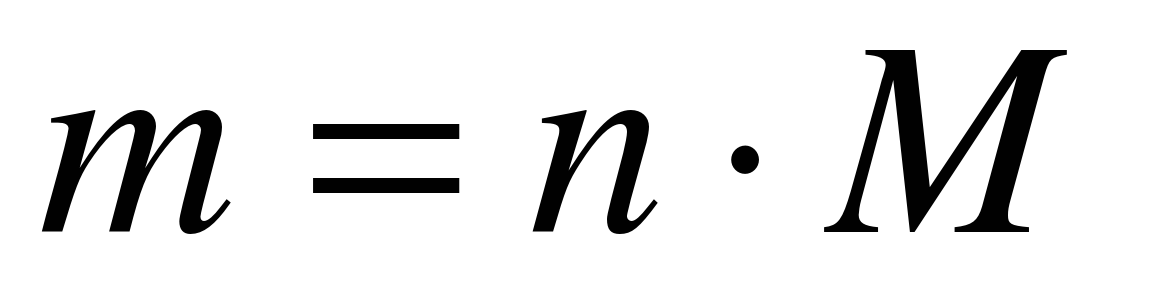
M (N 2) \u003d 28 g / mol
m (N 2) \u003d 10 mol * 28 g / mol
Answer: m (N 2) \u003d 280 g
Given:
n (N 2) \u003d 10 mol
Find:
m (N 2) - ?
(slide 24)V stage.Homework
§15, ex. 1-3
Answer the question:
How many sugar molecules are contained in a piece of refined sugar knowing that the mass of one piece is 1.3 g?
Mole, molar mass
AT chemical processes the smallest particles are involved - molecules, atoms, ions, electrons. The number of such particles, even in a small portion of matter, is very large. Therefore, to avoid mathematical operations with big numbers, to characterize the amount of a substance involved in chemical reaction, a special unit is used - mole.
A mole is such an amount of a substance that contains a certain number of particles (molecules, atoms, ions) equal to the Avogadro constant.
The Avogadro constant NA is defined as the number of atoms contained in 12 g of an isotope 12
FROM:
Thus, 1 mole of a substance contains 6.02 1023
particles of this substance.
Based on this, any amount of substance can be expressed by a certain number of moles ν (nu). For example, a sample of a substance contains 12.04 1023
molecules. Therefore, the amount of substance in this sample is:
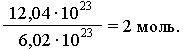
In general:
where N is the number of particles of a given substance;
N A- the number of particles that contains 1 mol of a substance (Avogadro's constant).
Molar mass substances (M)
is the mass that 1 mole of a given substance has.
This value, equal to the ratio of the mass m of a substance to the amount of substance ν, has the dimension kg/mol or g/mol. The molar mass, expressed in g/mol, is numerically equal to the relative relative molecular mass Mr (for substances atomic structure is the relative atomic mass of Ar).
For example, the molar mass of methane CH 4
is defined as follows:
Mr(CH 4
) = Ar(C) + 4 Ar(H) = 12+4 =16
M(CH 4 )=16 g/mol, i.e. 16 g CH4 contain 6.02 1023 molecules.
The molar mass of a substance can be calculated if its mass m and quantity (number of moles) ν are known, using the formula:
Accordingly, knowing the mass and molar mass of a substance, we can calculate the number of its moles:
or find the mass of a substance by the number of moles and the molar mass:
m = ν M
It should be noted that the value of the molar mass of a substance is determined by its qualitative and quantitative composition, i.e. depends on Mr and Ar. Therefore, different substances with the same number of moles have various masses m.
Example
Calculate masses of methane CH4
and ethane C2
H6
taken in the amount of ν = 2 mol each.
Solution
Molar mass of methane M(CH 4
) is equal to 16 g/mol;
molar mass of ethane M(C2
H6
) = 2 12+6=30 g/mol.
From here:
M(CH 4
) = 2 mol 16 g/mol = 32 g;
m(C2
H6
) \u003d 2 mol 30 g / mol \u003d 60 g.
Thus, a mole is a portion of a substance containing the same number of particles, but having a different mass for different substances, since particles of matter (atoms and molecules) are not the same in mass.
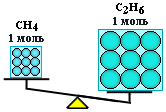
n(CH4 ) = n(С2 H6 ), but m(CH4 ) < m(С 2 H6 )
The calculation of ν is used in almost every computational problem.






