Quantum energy and wavelengths of various natural radiations
The article reveals the essence of the quantum properties of light. Talks about how they were discovered, and what it led to.
Planck and quantum
In the late nineteenth and early twentieth centuries, it was believed in scientific circles that absolutely everything was clear in physics. The most advanced knowledge at that time was Maxwell's equations and the study of various phenomena associated with electricity. Young people who aspired to do science were not recommended to go into physics: after all, there could only be routine studies that did not provide any breakthroughs. However, ironically, it was precisely this study of the properties of a long-familiar phenomenon that opened the way to new horizons of knowledge.
The wave and quantum properties of light began with the discovery of Max Planck. He studied the spectrum of an absolute black body and tried to find the most appropriate mathematical description of its radiation. As a result, he came to the conclusion that a certain minimum indivisible quantity, which he called the "quantum of action", should be introduced into the equation. And, since it was just a way to "cut the corner" for a simpler mathematical formula, he did not give this value any physical sense. However, other scientists, for example, A. Einstein and E. Schrödinger, noticed the potential of such a phenomenon as a quantum, and gave development to a new branch of physics.
I must say that Planck himself did not fully believe in the fundamental nature of his discovery. The scientist, trying to refute the quantum properties of light, briefly rewrote his formula, indulging in various mathematical tricks to get rid of this quantity. But nothing came of it: the genie had already been let out of the bottle.
Light is a quantum of the electromagnetic field
After Planck's discovery known fact that light has wave properties, supplemented by another: a photon is a quantum electromagnetic field. That is, light consists of very small, indivisible packets of energy. Each of these packets (photon) is characterized by frequency, wavelength and energy, and all these quantities are interconnected. The speed of light in a vacuum is the fastest in the known universe, at about 300,000 kilometers per second.

It should be noted that other quantities are also quantized (that is, they are divided into the smallest indivisible parts):
- gluon field;
- gravitational field;
- collective motions of crystal atoms.
Quantum: difference from electron
You should not think that in each type of field there is a certain smallest quantity, which is called a quantum: in the electromagnetic scale there are both very small and high-energy waves (for example, X-rays), and very large, but at the same time “weak” ones (for example, radio waves ). It's just that each quantum travels through space as a whole. Photons, it is worth noting, are able to lose some of their energy when interacting with insurmountable potential barriers. This phenomenon is called "tunneling".
The interaction of light and matter
After such a bright opening, questions rained down:
- What happens to a quantum of light when it interacts with matter?
- Where does the energy carried by a photon go when it collides with a molecule?
- Why can one wavelength be absorbed and another wavelength emitted?

The main thing is that the phenomenon of light pressure has been proven. This fact gave new occasion for reflection: so the photon had momentum and mass. The corpuscular-wave dualism of microparticles adopted after that greatly facilitated the understanding of the madness happening in this world: the results did not fit into any logic that existed before.
Energy transfer
Further research only confirmed the quantum properties of light. The photoelectric effect showed how the energy of a photon is transferred to matter. Along with reflection and absorption, illumination is capable of pulling electrons from the surface of a body. How does this happen? The photon transfers its energy to the electron, which becomes more mobile and gains the ability to overcome the force of bonding with the nuclei of matter. The electron leaves its native element and rushes somewhere outside the familiar environment.
Types of photoelectric effect
The phenomenon of the photoelectric effect, which confirms the quantum properties of light, has different types and depends on which solid photon collides. If it collides with a conductor, then the electron leaves the substance, as already described above. This is the essence external photoelectric effect.
But if a semiconductor or dielectric is illuminated, then the electrons do not leave the body, but are redistributed, facilitating the movement of charge carriers. Thus, the phenomenon of improving conductivity when illuminated is called the intrinsic photoelectric effect.
External photoelectric formula
Oddly enough, but the internal photoelectric effect is very difficult to understand. It is necessary to know the band theory of the field, to understand the transitions through the band gap and to understand the essence of the electron-hole conductivity of semiconductors in order to fully realize the importance of this phenomenon. In addition, the internal photoelectric effect is not so often used in practice. Confirming the quantum properties of light, the formulas for the external photoelectric effect limit the layer from which light is able to pull out electrons.
where h is Planck's constant, ν is a quantum of light of a certain wavelength, A is the work that an electron does to leave matter, W is the kinetic energy (and hence the speed) with which it flies out.
Thus, if all the energy of a photon is spent only on the exit of an electron from the body, then on the surface it will have zero kinetic energy and can't really get out. Thus, the internal photoelectric effect also takes place in a sufficiently thin external word of the illuminated substance. This severely limits its application.

There is a possibility that an optical quantum computer will still use the internal photoelectric effect, but such technology does not yet exist.
Laws of the external photoelectric effect
At the same time, the quantum properties of light are not entirely useless: the photoelectric effect and its laws make it possible to create a source of electrons. While these laws were formulated in full by Einstein (for which he received the Nobel Prize), various prerequisites arose much earlier than the twentieth century. The appearance of a current when an electrolyte was illuminated was first observed already at the beginning of the nineteenth century, in 1839.

There are three laws in total:
- The strength of the saturation photocurrent is proportional to the intensity of the light flux.
- The maximum kinetic energy of electrons leaving a substance under the action of photons depends on the frequency (and hence the energy) of the incident radiation, but does not depend on the intensity.
- Each substance with the same type of surface (smooth, convex, rough, porous) has a red border of the photoelectric effect. That is, there is such a smallest energy (and hence the frequency) of a photon, which still detaches electrons from the surface.
All these patterns are logical, but they should be considered in more detail.
Explanation of the laws of the photoelectric effect
The first law means the following: the more photons fall on one square meter of surface area per second, the more electrons this light is able to “take away” from the illuminated substance.

Basketball is an example: the more often a player throws the ball, the more often he will hit. Of course, if the player is good enough and not injured during the match.
The second law actually gives the frequency response of the emitted electrons. The frequency and wavelength of a photon determine its energy. Red light has the lowest energy in the visible spectrum. And no matter how many red photons the lamp sends to matter, they are able to transfer only low energy to electrons. Therefore, even if they were torn from the surface itself and did almost no work of exit, then their kinetic energy cannot be higher than a certain threshold. But if we illuminate the same substance with violet rays, then the speed of the fastest electrons will be much higher, even if there are very few violet quanta.
The third law has two components - the red border and the state of the surface. Many factors depend on whether the metal is polished or rough, whether it has pores or whether it is smooth: how many photons will be reflected, how they will be redistributed over the surface (obviously, less light will get into the pits). So you can compare different substances with each other only with the same surface condition. But the energy of a photon, which is still able to tear off an electron from a substance, depends only on the type of substance. If the nuclei do not attract charge carriers very strongly, then the photon energy can be lower, and, consequently, the red border is deeper. And if the nuclei of a substance hold their electrons tightly and do not want to part with them so easily, then the red border shifts to the green side.
Electromagnetic radiation with energies up to 250 keV is commonly called x-rays
, and above that - g radiation
. The radiation of radioactive isotopes, regardless of energy, is usually denoted as
g-rays
.
All other types of AI have a corpuscular nature, representing elementary particles. The mechanism of energy transfer of all charged particles is approximately the same. When passing through matter, a charged particle loses its energy, causing ionization and excitation of atoms until the total energy supply decreases to such an extent that the particle loses its ionizing ability and is usually captured by some atom to form an ion.
The energy lost by a charged particle per unit of its path is called linear energy loss. Depending on this, all ionizing radiation is divided into rarely- and densely ionizing . Rarely ionizing radiation includes all types of electromagnetic radiation and electrons, and densely ionizing radiation includes protons, deuterons and heavier particles.
The nature of the emitted radiation was studied by its absorption in matter and by the deflection of these rays in a magnetic and electric field.
In 1899, E. Rutherford, studying the behavior of radioactive radiation in an electric field, found that it consists of two components (see Fig. 11).
Rice. 11. Rutherford's experience.
The first of them slightly deviates towards the negatively charged plate, and the other strongly deviates towards the positively charged plate. These components he called alpha rays and beta rays. Since most of the space in an atom is empty, fast a-particles can almost freely penetrate significant layers of matter containing several thousand layers of atoms.
The scattering of charged particles observed by Rutherford is explained by such a distribution of charges in the atom. In collisions with individual electrons, a-particles deviate by very small angles, since the mass of the electron is small. However, on those rare occasions when it flies by close range from one of the atomic nuclei, under the influence of a strong electric field core can be deflected to a large angle.
A year later, P. Willard found that the composition of radioactive radiation also includes a third component: gamma rays, which are not deflected by either magnetic or electric fields. It was found that radioactive nuclei can emit particles of three types: positively and negatively charged and neutral. Until the nature of these radiations was clarified, the rays that deviated towards the negatively charged plate were conditionally called alpha particles , deviated towards a positively charged plate - beta rays , and the rays that did not deviate at all were called gamma rays (Fig. 12.).

Rice. 12. Components of radioactive radiation.
K - lead container, R - radioactive preparation,
Ф – photographic plate, – magnetic field.
Alpha particles (a) are the nuclei of the helium atom and consist of two protons and two neutrons. They have double positive charge and a relatively large mass equal to 4.0003 a.m.u.
For each isotope, the energy of alpha particles is constant. The range of alpha particles in air is, depending on the energy, 2–10 cm, and in biological tissues it is several tens of microns. Since alpha particles are massive and have high energy, their path in matter is straightforward; they cause strongly pronounced effects of ionization and fluorescence. Alpha radiation when it enters the human body is extremely dangerous, since all the energy of a-particles is transferred to the cells of the body.
Beta radiation (b) represents the stream of particles (electrons or positrons) emitted by nuclei during beta decay. The physical characteristic of electrons of nuclear origin is the same as that of electrons atomic shell. Beta particles are denoted by the symbol b - (electronic decay), b + (positron decay).
Unlike alpha particles, beta particles of the same radioactive element have different amounts of energy. This is explained by the fact that during beta decay, a beta particle and a neutrino are emitted from the atomic nucleus simultaneously. The energy released during each decay event is distributed between the beta particle and the neutrino. This is an electrically neutral particle that moves at the speed of light, has no rest mass and has a large penetrating power; making it difficult to register. If a b-particle is emitted with a large amount of energy, then a neutrino is emitted with a low energy level and vice versa. The range of beta particles in the same medium is not the same. The path in the substance of such particles is tortuous, they easily change the direction of movement under the action of the electric fields of oncoming atoms. Beta particles have less of an ionizing effect than alpha particles. Their range in the air can be up to 25 cm, and in biological tissues - up to 1 cm. Various radioactive isotopes differ in the energy of beta particles. Their maximum energy has wide limits from 0.015–0.05 MeV (soft beta radiation) to 3–12 MeV (hard beta radiation).
Gamma radiation (g) is a stream of electromagnetic waves; it's like radio waves, visible light, ultraviolet and infrared rays, and x-rays.

Rice. 13. Scheme of the formation of gamma radiation
Different kinds radiation differ in the conditions of formation and certain properties. X-ray radiation arises when fast electrons decelerate in the electric field of the nucleus of atoms of a substance (bremsstrahlung X-rays) or during rearrangement electron shells atoms during ionization and excitation of atoms and molecules (characteristic x-ray radiation). During various transitions from an excited state to an unexcited state, emission can occur visible light, infrared and ultraviolet rays. Gamma quanta are emitted by the nuclei of atoms during the alpha and beta decay of natural and artificial radionuclides in those cases when an excess of energy is found in the daughter nucleus that is not captured by corpuscular radiation. Gamma rays have no rest mass, no charge, and therefore do not deviate in an electric or magnetic field. In matter and in vacuum, gamma radiation propagates in a straight line and uniformly in all directions. The energy of a gamma quantum is proportional to the oscillation frequency and is determined by the formula:
Еg = h × ν, (1.16)
where h is Planck's universal constant (4.13 × 10 –21 MeV/s); n is the frequency of oscillations per second.
The oscillation frequency is related to the wavelength. The longer the wavelength, the lower the oscillation frequency and vice versa, i.e. the frequency is inversely proportional to the wavelength. Energy gamma radiation ranges from a few keV to 2–3 MeV. The composition of the gamma radiation flux often includes quanta of various energy values. However, their set is constant for each isotope.
Gamma quanta, having no charge and rest mass, cause a weak ionizing effect, but have a high penetrating power. The path in the air reaches 100–150 m (see Fig. 14).

Rice. 14. Penetrating ability of alpha, beta and gamma particles.
Neutrons. Unlike charged particles, neutrons do not carry electric charge, which allows them to freely penetrate deep into the atoms; colliding with the latter, they are either absorbed by it or repelled. As a result of elastic scattering, strongly ionizing protons of high energy are formed, and when neutrons are absorbed atomic nuclei protons, alpha particles and g-quanta fly out of the latter, which also produce ionization. Thus, under neutron irradiation, the final biological effect is associated with ionization produced indirectly by secondary particles or g-quanta. The contribution of one or another nuclear interaction of neutrons depends on the composition of the irradiated substance and on their energy. According to the energy value, four types of neutrons are distinguished: fast, intermediate, slow and thermal (see Fig. 15).
Neutrons are classified as densely ionizing radiation, since the range of the recoil protons they form is small. However, they occur at great depths due to the high penetrating power of neutrons.
Negative p mesons- negatively charged particles with a mass 273 times the mass of an electron. They are obtained by artificial methods. These particles have a unique ability to interact with the nuclei of atoms. Negative pimesons with energies on the order of 25–100 MeV travel all the way through matter until complete deceleration with almost no nuclear interactions. At the end of the run, they are captured with 100% probability by the nuclei of tissue atoms.

Rice. 15. Types of neutrons.
1.3.2. Interaction of radioactive radiations
with substance
First, purely physical the stage of interaction, which takes place in millionths of a second, consists in the transfer of a part of the photon energy to one of the electrons of the atom, followed by ionization and excitation. Ions and excited atoms, which have excess energy, are therefore characterized by increased chemical reactivity, they are able to enter into reactions that are not possible for ordinary, unexcited atoms.
Second, physical and chemical, the stage proceeds depending on the composition and structure of the irradiated substance. Of fundamental importance is the presence of water and oxygen. If they are absent, then the possibilities of chemical interaction of atoms activated by radiation are limited, localized.
Interaction of alpha and beta particles. Charged particles, passing through matter, gradually lose energy as a result of interaction with the electrons of atoms, as well as with the electric field of the nucleus. The kinetic energy of a- and b-particles is wasted on ionization, that is, on the detachment of electrons from an atom, and on the excitation of atoms and molecules. Interacting with the electric field of the nucleus, the charged particle slows down and changes the direction of its movement, while emitting radiation, which in its characteristics is close to X-ray and is called bremsstrahlung X-ray.
The quantity that determines the energy side of the ionization process is ionization work – average work spent on the formation of one pair of ions. Charged particles, different in nature, but with the same energy, form almost the same number of pairs of ions. However ionization density , i.e. the number of pairs of ions per unit path of a particle in a substance will be different. The ionization density increases with an increase in the charge of the particle and with a decrease in its velocity.
Passing through matter, charged particles gradually lose energy and speed, so the ionization density along the path of the particle increases and reaches a value at the end of the path. At the end of the path, the a-particle attaches two electrons to itself and turns into a helium atom, and
b-particle (electron) can be included in one of the atoms of the medium.
The path traveled by an a- or b-particle in a substance, during which it produces ionization, is called particle range . The range of an alpha particle in air can reach 10 cm, and in soft biological tissue - several tens of microns. The range of beta particles in the air reaches 25 m, and in tissues up to 1 cm.
Alpha particles propagate in matter in a straight line and change direction only when they collide with the nuclei of oncoming atoms. Beta particles, having a small mass, high speed and negative charge, deviate significantly from the original direction as a result of collisions with orbiting electrons and nuclei of oncoming atoms (scattering effect). By undergoing multiple scattering, beta particles can even move in the opposite direction - backscattering. Due to the significant scattering of b-particles, the true path length in matter is 1.5–4 times greater than their range. Another difference is in the passage of a- and b-particles through matter. Since all alpha particles emitted by an isotope have a relatively equal energy and move rectilinearly in the substance, then their number in the beam passing through unit surface of the absorber drops sharply to zero only at the end of the path. The spectrum of beta particles is continuous, therefore, with an increase in the thickness of the absorber, the number of beta particles in a beam passing through a unit surface decreases gradually.
The weakening of the intensity of the flow of b-particles in matter approximately obeys the exponential dependence:
N \u003d N 0 × e - m a, (1.17)
where N is the number of beta particles that have passed through the absorber layer d cm, N 0 is the number of beta particles arriving in 1 s at the absorber area equal to 1 cm 2; e - base natural logarithms; m is the linear radiation attenuation coefficient characterizing the relative attenuation of the intensity of the b-particle flux after passing through an absorber 1 cm thick.
Interaction of gamma radiation with matter. During the radioactive decay of a nucleus, g-quanta with different energies are emitted. When passing through matter, they lose energy practically due to three effects: photoelectric absorption, Compton scattering, and the formation of electron-positron pairs.
At photoelectric effect the energy of the incident quantum is completely absorbed by the substance, as a result, free electrons appear that have a certain kinetic energy, the value of which is equal to the energy of the radiation quantum minus the work function of the given electron from the atom. A free electron, associating with one of the neutral atoms, generates a negative ion. The photoelectric effect is characteristic only for long-wavelength X-rays. Its probability depends on the atomic number and is proportional to Z 5 . The process of the photoelectric effect is impossible on weakly bound and free electrons (not bound to the nucleus), since they cannot absorb g-quanta.
At Compton effect g-quanta, colliding with electrons, transfer to them not all of their energy, but only part of it, and after the collision change their direction of motion. The electrons formed as a result of collision with g-quanta acquire significant kinetic energy and waste it on the ionization of matter (secondary ionization). That. as a result of the Compton effect, the intensity of gamma radiation is weakened due to the fact that g-quanta, interacting with the electrons of the medium, scatter in different directions and go beyond the primary beam, as well as due to the transfer of part of their energy to the electrons.
Pairing. Some g-quanta with an energy of at least 1.02 MeV, passing through matter, are converted under the action of a strong electric field near the nucleus into an electron-positron pair. In this case, there is a transition from one form of matter - gamma radiation to another - into particles of matter. The formation of such a pair of particles is possible only at photon energies not less than the energy equivalent to the mass of both particles - an electron and a positron.
The resulting electron-positron pair subsequently disappears, turning into two secondary g-quanta with an energy equal to the energy equivalent of the rest mass of particles - 0.511 MeV. The probability of pair formation increases with an increase in the energy of g-quanta and in the density of the absorber.
The law of attenuation of gamma radiation by matter differs significantly from the law of attenuation of a- and b-particles. The g-ray beam is absorbed continuously as the thickness of the absorber increases. Those. Whatever the thickness of the substance layer, it is impossible to completely absorb the flow of g-rays, but only to weaken its intensity by any given number of times. This is the essential difference between the nature of the attenuation of g-rays and the attenuation of a- and b-particles, for which it is always possible to choose a layer of matter in which the flux of a- or b-particles is completely absorbed.
The g-ray beam attenuation law has the following form:
I \u003d I 0 × e - m a, (1.18)
where I is the intensity of the g-ray beam that has passed through the absorber layer; I 0 is the intensity of the incident beam of gamma rays; m is the linear attenuation coefficient, equal to the relative decrease in the intensity of the gamma-ray beam after passing through the absorber layer 1 cm thick. The linear attenuation coefficient is the total coefficient that takes into account the attenuation of the gamma-ray beam due to all three processes: photoelectric effect (t f), Compton effect (t k) and pair formation (t p):
m \u003d t f + t k + t p (1.19)
Section 2 (lectures #3–4)
FUNDAMENTALS OF RADIOECOLOGY
In physics for grade 11 (Kasyanov V.A., 2002),
a task №87
to chapter " Quantum theory of electromagnetic radiation. MAIN PROVISIONS».
thermal radiation
Completely black body
thermal radiation- electromagnetic radiation emitted by heated bodies due to its internal energy.
Completely black body- a body that absorbs all the energy of radiation incident on it of any frequency at an arbitrary temperature.
Spectral density of energy luminosity is the energy of electromagnetic radiation emitted per unit of time per unit area of the body surface in a unit frequency interval. Unit of spectral density of energy luminosity J/m 2 . The energy of a radiation quantum is directly proportional to the frequency v of the radiation:
where h = 6.6 10 -34 J s is Planck's constant.
Photon- microparticle, quantum of electromagnetic radiation.
Laws thermal radiation: Wien's Displacement Law
 where λm is the wavelength at which the maximum spectral density of the blackbody energy luminosity falls, T is the temperature of the blackbody, b ≈ 3000 µm K is Wien's constant.
where λm is the wavelength at which the maximum spectral density of the blackbody energy luminosity falls, T is the temperature of the blackbody, b ≈ 3000 µm K is Wien's constant.
Stefan-Boltzmann law: The integral luminosity of a black body is proportional to the fourth power of its absolute temperature:
 where σ =
5.67 10 -8 W / (m 2 K 4) - Stefan-Boltzmann constant.
where σ =
5.67 10 -8 W / (m 2 K 4) - Stefan-Boltzmann constant.
photoelectric effect the phenomenon of ejection of electrons from solid and liquid substances under the action of light.
Laws of the photoelectric effect
1. The saturation photocurrent is directly proportional to the intensity of the light incident on the cathode.
2. The maximum kinetic energy of photoelectrons is directly proportional to the frequency of light and does not depend on its intensity.
3. For each substance there is a minimum frequency of light, called the red limit of the photoelectric effect, below which the photoelectric effect is impossible.
Einstein's equation for the photoelectric effect:
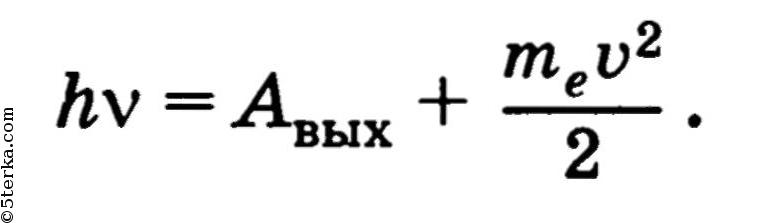 The energy of the photon is used to perform the work function and to communicate the kinetic energy to the emitted photoelectron. The work function is the minimum work that must be done to remove an electron from a metal.
The energy of the photon is used to perform the work function and to communicate the kinetic energy to the emitted photoelectron. The work function is the minimum work that must be done to remove an electron from a metal.
red border photo effect

Corpuscular-wave dualism - manifestation in the behavior of the same object of both corpuscular and wave properties. Corpuscular-wave dualism is a universal property of any material objects.
wave theory correctly describes the properties of light at high intensities, i.e. when the number of photons is large.
Quantum theory is used to describe the properties of light at low intensities, i.e. when the number of photons is small.
Any particle with momentum p Answer the de Broglie wavelength is:

The state of the micro-object changes during the measurement process. Simultaneous precise determination of the position and momentum of a particle is impossible.
Heisenberg uncertainty relations:
1. The product of the uncertainty of the particle's coordinate and the uncertainty of its momentum is not less than Planck's constant:
 2. The product of the uncertainty of the energy of a particle and the uncertainty of the time of its measurement is not less than Planck's constant:
2. The product of the uncertainty of the energy of a particle and the uncertainty of the time of its measurement is not less than Planck's constant:
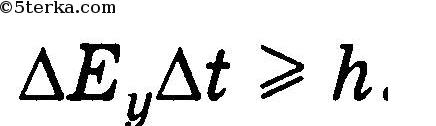
Bohr's postulates:
1. In a stable atom, an electron can only move along special, stationary orbits, without radiating electromagnetic energy
2. The emission of light by an atom occurs during the transition of an atom from a stationary state with a higher energy E k to a stationary state with a lower energy Е n . The energy of the emitted photon is equal to the difference between the energies of the stationary states:

Bohr's orbit quantization rule:
On the circumference of each stationary orbit fits an integer n of de Broglie wavelengths, with Answer corresponding to the motion of an electron

Ground state of the atom is the state of minimum energy.
Luminescence- non-equilibrium radiation of matter.
Spectral analysis- a method for determining the chemical composition and other characteristics of a substance by its spectrum.
Basic radiative processes of atoms: absorption of light, spontaneous and stimulated emission.
light absorption is accompanied by the transition of the atom from the ground state to the excited state.
Spontaneous emission- radiation emitted during the spontaneous transition of an atom from one state to another.
stimulated emission- radiation of an atom that occurs when it passes to a lower energy level under the influence of external electromagnetic radiation.
Laser- source of radiation amplified as a result of induced radiation.
Inverse population of energy levels- non-equilibrium state of the medium, in which the concentration of atoms in the excited state is greater than the concentration of atoms in the ground state.
Metastable state- the excited state of the atom, in which it can be much longer than in other states.






