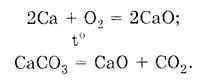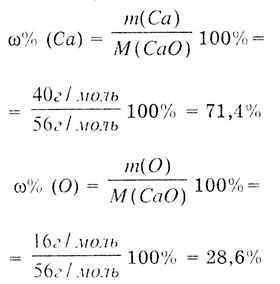Law of conservation of mass in chemistry wording. The law of conservation of mass and energy. The greatest achievement of world science
The law of conservation of mass.
The mass of substances entering into a chemical reaction is equal to the mass of substances formed as a result of the reaction.
The law of conservation of mass is a special case of the general law of nature - the law of conservation of matter and energy. Based on this law, chemical reactions can be displayed using chemical equations, using the chemical formulas of substances and stoichiometric coefficients that reflect the relative quantities (number of moles) of the substances involved in the reaction.
For example, the combustion reaction of methane is written as follows:
The law of conservation of mass of substances
(M.V. Lomonosov, 1748; A. Lavoisier, 1789)
The mass of all substances involved in a chemical reaction is equal to the mass of all the products of the reaction.
The atomic-molecular theory explains this law as follows: as a result of chemical reactions, atoms do not disappear and do not arise, but they are rearranged (i.e., a chemical transformation is the process of breaking some bonds between atoms and the formation of others, as a result of which from the molecules of the original substances, molecules of reaction products are obtained). Since the number of atoms before and after the reaction remains unchanged, their total mass should also not change. Mass was understood as a quantity characterizing the amount of matter.
At the beginning of the 20th century, the formulation of the law of conservation of mass was revised in connection with the advent of the theory of relativity (A. Einstein, 1905), according to which the mass of a body depends on its speed and, therefore, characterizes not only the amount of matter, but also its movement. The energy E received by the body is related to the increase in its mass m by the relation E = m c 2 , where c is the speed of light. This ratio is not used in chemical reactions, because 1 kJ of energy corresponds to a mass change of ~10 -11 g and m can hardly be measured. AT nuclear reactions, where Е is ~10 6 times greater than in chemical reactions, m should be taken into account.
Based on the law of conservation of mass, it is possible to draw up equations for chemical reactions and use them to make calculations. It is the basis of quantitative chemical analysis.
Law of constancy of composition
Composition constancy law ( J.L. Proust, 1801 -1808.) - any specific chemically pure compound, regardless of the method of its preparation, consists of the same chemical elements, and the ratios of their masses are constant, and relative numbers them atoms expressed as whole numbers. This is one of the fundamental laws chemistry.
The law of constancy of composition does not hold for berthollids(compounds of variable composition). However, conventionally, for simplicity, the composition of many berthollides is recorded as constant. For example, the composition iron(II) oxide is written as FeO (instead of the more precise formula Fe 1-x O).
|
THE LAW OF CONSTANT COMPOSITION |
|
According to the law of constancy of composition, any pure substance has a constant composition, regardless of the method of its preparation. So, calcium oxide can be obtained in the following ways:
Regardless of how the CaO substance is obtained, it has a constant composition: one calcium atom and one oxygen atom form the calcium oxide CaO molecule. Determine the molar mass of CaO:
We determine the mass fraction of Ca by the formula:
Conclusion: In chemically pure oxide, the mass fraction of calcium is always 71.4% and oxygen 28.6%. |
Law of multiple ratios
The law of multiple ratios is one of stoichiometric laws chemistry: if two substances (simple or difficult) form more than one compound with each other, then the masses of one substance per the same mass of another substance are related as whole numbers, usually small.
Examples
1) The composition of nitrogen oxides (in percent by mass) is expressed by the following numbers:
|
Nitrous oxide N 2 O |
Nitric oxide NO |
Nitrous anhydride N 2 O 3 |
Nitrogen dioxide NO 2 |
Nitric anhydride N 2 O 5 |
|
|
Private O/N |
Dividing the bottom row numbers by 0.57, we see that they are related as 1:2:3:4:5.
2) Calcium chloride forms with water 4 crystalline hydrate, the composition of which is expressed by the formulas: CaCl 2 H 2 O, CaCl 2 2H 2 O, CaCl 2 4H 2 O, CaCl 2 6H 2 O, i.e. in all these compounds, the mass of water per CaCl molecule 2 are related as 1:2:4:6.
Law of Volumetric Relations
(Gay-Lussac, 1808)
"The volumes of gases entering into chemical reactions and the volumes of gases formed as a result of the reaction are related to each other as small integers."
Consequence. Stoichiometric coefficients in the equations of chemical reactions for molecules of gaseous substances show in what volume ratios gaseous substances react or are obtained.
2CO + O 2 2CO 2
When two volumes of carbon monoxide (II) are oxidized with one volume of oxygen, 2 volumes of carbon dioxide are formed, i.e. the volume of the initial reaction mixture is reduced by 1 volume.
b) In the synthesis of ammonia from the elements:
n 2 + 3h 2 2nh 3
One volume of nitrogen reacts with three volumes of hydrogen; in this case, 2 volumes of ammonia are formed - the volume of the initial gaseous reaction mass will decrease by 2 times.
Klaiperon-Mendeleev equation
If we write the combined gas law for any mass of any gas, then we get the Claiperon-Mendeleev equation:
where m is the mass of gas; M is the molecular weight; p - pressure; V - volume; T - absolute temperature (°K); R is the universal gas constant (8.314 J / (mol K) or 0.082 l atm / (mol K)).
For a given mass of a particular gas, the m/M ratio is constant, so the combined gas law is derived from the Claiperon-Mendeleev equation.
What volume will take up at a temperature of 17 ° C and a pressure of 250 kPa carbon monoxide (II) weighing 84 g?
The number of moles of CO is:
(CO) \u003d m (CO) / M (CO) \u003d 84 / 28 \u003d 3 mol
CO volume at n.c. is
3 22.4 l = 67.2 l
From the combined gas law of Boyle-Mariotte and Gay-Lussac:
(P V) / T = (P 0 V 0) / T 2
V (CO) \u003d (P 0 T V 0) / (P T 0) \u003d (101.3 (273 + 17) 67.2) / (250 273) \u003d 28.93 l
The relative density of gases shows how many times 1 mole of one gas is heavier (or lighter) than 1 mole of another gas.
D A(B) = (B) (A) = M (B) / M (A)
The average molecular weight of a mixture of gases is equal to the total mass of the mixture divided by the total number of moles:
M cf \u003d (m 1 + .... + m n) / ( 1 + .... + n) \u003d (M 1 V 1 + .... M n V n) / ( 1 + .. .. + n)
LAW OF ENERGY CONSERVATION : in isolation. the energy of the system remains constant, only transitions of one type of energy into another are possible. In the thermodynamics of energy conservation, the law corresponds to the first law of thermodynamics, which is expressed by the equation Q \u003d DU + W, where Q is the number of heat communicated to the system, DU is the change in ext. energy of the system, W is the work done by the system. A special case of the conservation of energy law is the Hessian law.
The concept of energy was revised in connection with the advent of the theory of relativity (A. Einstein, 1905): the total energy E is proportional to the mass m and is related to it by the relation E = mc2, where c is the speed of light. Therefore, the mass can be expressed in units of energy and formulate a more general law of conservation of mass and energy: in iso-lyre. In a system, the sum of masses and energy is constant, and only transformations in strictly equivalent ratios of some forms of energy into others and equivalently related changes in mass and energy are possible.
Law of Equivalents
substances interact with each other in amounts proportional to their equivalents. When solving some problems, it is more convenient to use a different formulation of this law: the masses (volumes) of substances reacting with each other are proportional to their equivalent masses (volumes).
equivalents: chemical elements combine with each other in strictly defined quantities corresponding to their equivalents. The mathematical expression of the law of equivalents is as follows: where m1 and m2 are the masses of reacting or formed substances, m eq (1) and m eq (2) are the equivalent masses of these substances.
For example: a certain amount of metal, the equivalent mass of which is 28 g / mol, displaces 0.7 liters of hydrogen measured under normal conditions from an acid. Determine the mass of the metal. Solution: knowing that the equivalent volume of hydrogen is 11.2 l / mol, it is a proportion: 28 g of metal are equivalent to 11.2 liters of hydrogen x g of metal are equivalent to 0.7 liters of hydrogen. Then x \u003d 0.7 * 28 / 11.2 \u003d 1.75 g.
To determine the equivalent or equivalent mass, it is not necessary to proceed from its combination with hydrogen. They can be determined by the composition of the compound of a given element with any other, the equivalent of which is known.
For example: when 5.6 g of iron was combined with sulfur, 8.8 g of iron sulfide was formed. It is necessary to find the equivalent mass of iron and its equivalent, if it is known that the equivalent mass of sulfur is 16 g/mol. Solution: from the conditions of the problem it follows that in iron sulfide, 5.6 g of iron accounts for 8.8-5.6 = 3.2 g of sulfur. According to the law of equivalents, the masses of interacting substances are proportional to their equivalent masses, that is, 5.6 g of iron is equivalent to 3.2 g of sulfur meq (Fe) is equivalent to 16 g/mol of sulfur. It follows from here that m3KB(Fe) = 5.6*16/3.2=28 g/mol. The iron equivalent is: 3=meq(Fe)/M(Fe)=28 g/mol:56 g/mol=1/2. Therefore, the equivalent of iron is 1/2 mole, that is, 1 mole of iron contains 2 equivalents.
Avogadro's law
Consequences of the law
The first corollary of Avogadro's law: one mole of any gas under the same conditions occupies the same volume.
In particular, under normal conditions, i.e. at 0 ° C (273 K) and 101.3 kPa, the volume of 1 mole of gas is 22.4 liters. This volume is called the molar volume of gas V m . You can recalculate this value to other temperatures and pressures using the Mendeleev-Clapeyron equation:
![]() .
.
The second corollary of Avogadro's law: the molar mass of the first gas is equal to the product molar mass of the second gas by the relative density of the first gas according to the second.
This position was of great importance for the development of chemistry, since it makes it possible to determine the partial weight of bodies capable of passing into a gaseous or vaporous state. If through m we denote the partial weight of the body, and through d is its specific gravity in the vapor state, then the ratio m / d should be constant for all bodies. Experience has shown that for all the bodies studied, passing into steam without decomposition, this constant is equal to 28.9, if, when determining the partial weight, we proceed from the specific gravity of air, taken as a unit, but this constant will be equal to 2, if we take the specific gravity of hydrogen as a unit. Denoting this constant, or, what is the same, the partial volume common to all vapors and gases through FROM, we have from the formula on the other hand m = dC. Since the specific gravity of steam is easily determined, then, substituting the value d in the formula, the unknown partial weight of the given body is also displayed.
Thermochemistry
thermal effect chemical reaction
From Wikipedia, the free encyclopedia
Thermal effect of a chemical reaction or change enthalpy system due to the occurrence of a chemical reaction - the amount of heat related to the change in the chemical variable received by the system in which the chemical reaction took place and the reaction products took the temperature of the reactants.
In order for the thermal effect to be a quantity that depends only on the nature of the ongoing chemical reaction, the following conditions must be met:
The reaction must proceed either at a constant volume Q v (isochoric process), or at constant pressure Q p( isobaric process).
No work is done in the system, except for the expansion work that is possible with P = const.
If the reaction is carried out under standard conditions at T \u003d 298.15 K \u003d 25 ° C and P \u003d 1 atm \u003d 101325 Pa, the thermal effect is called the standard thermal effect of the reaction or standard enthalpy reactions Δ H r O . In thermochemistry, the standard thermal effect of a reaction is calculated using the standard enthalpies of formation.
Standard enthalpy of formation (standard heat of formation)
The standard heat of formation is understood as the heat effect of the reaction of formation of one mole of a substance from simple substances, its components, which are in stable standard states.
For example, the standard enthalpy of formation is 1 mol methane from carbon and hydrogen equal to the heat of the reaction:
C (tv) + 2H 2 (g) \u003d CH 4 (g) + 76 kJ / mol.
The standard enthalpy of formation is denoted Δ H fO . Here the index f means formation (education), and the crossed out circle, resembling the Plimsol disk - that the value refers to standard state substances. In the literature, another designation for the standard enthalpy is often found - ΔH 298,15 0 , where 0 indicates the pressure is equal to one atmosphere (or, somewhat more precisely, to the standard conditions ), and 298.15 is the temperature. Sometimes index 0 is used for quantities related to pure substance, stipulating that it is possible to designate standard thermodynamic quantities with it only when it is a pure substance that is chosen as the standard state . The standard can also be taken, for example, the state of matter in extremely dilute solution. "Plimsol disk" in this case means the actual standard state of matter, regardless of its choice.
The enthalpy of formation of simple substances is assumed to be zero, and the zero value of the enthalpy of formation refers to the state of aggregation, which is stable at T = 298 K. For example, for iodine in the crystalline state Δ H I2(tv) 0 = 0 kJ/mol, and for liquid iodine Δ H I2(l) 0 = 22 kJ/mol. The enthalpies of formation of simple substances under standard conditions are their main energy characteristics.
The thermal effect of any reaction is found as the difference between the sum of the heats of formation of all products and the sum of the heats of formation of all reactants in this reaction (corollary Hess' law):
Δ H reactions O = ΣΔ H f O (products) - ΣΔ H f O (reagents)
Thermochemical effects can be included in chemical reactions. Chemical Equations in which the amount of released or absorbed heat is indicated, are called thermochemical equations. Reactions accompanied by the release of heat into the environment have a negative thermal effect and are called exothermic. Reactions accompanied by the absorption of heat have a positive thermal effect and are called endothermic. The thermal effect usually refers to one mole of the reacted starting material, the stoichiometric coefficient of which is maximum.
Temperature dependence of the thermal effect (enthalpy) of the reaction
To calculate the temperature dependence of the enthalpy of reaction, it is necessary to know the molar heat capacity substances involved in the reaction. The change in the enthalpy of the reaction with increasing temperature from T 1 to T 2 is calculated according to the Kirchhoff law (it is assumed that in this temperature range the molar heat capacities do not depend on temperature and there is no phase transformations):
![]()
If phase transformations occur in a given temperature range, then in the calculation it is necessary to take into account the heats of the corresponding transformations, as well as the change in the temperature dependence of the heat capacity of substances that have undergone such transformations:
where ΔC p (T 1 ,T f) is the change in heat capacity in the temperature range from T 1 to the phase transition temperature; ΔC p (T f ,T 2 ) is the change in heat capacity in the temperature range from the phase transition temperature to the final temperature, and T f is the phase transition temperature.
Standard enthalpy of combustion
Standard enthalpy of combustion - Δ H Gor o, the thermal effect of the reaction of combustion of one mole of a substance in oxygen to the formation of oxides in the highest degree of oxidation. The heat of combustion of non-combustible substances is assumed to be zero.
Standard enthalpy of dissolution
Standard enthalpy of dissolution - Δ H solution, the thermal effect of the process of dissolving 1 mole of a substance in an infinitely large amount of solvent. Composed of the heat of destruction crystal lattice and warmth hydration(or heat solvation for non-aqueous solutions), released as a result of the interaction of solvent molecules with molecules or ions of the dissolved substance with the formation of compounds of variable composition - hydrates (solvates). The destruction of the crystal lattice, as a rule, is an endothermic process - Δ H resh > 0, and ion hydration is exothermic, Δ H hydra< 0. В зависимости от соотношения значений ΔH resh and Δ H hydr enthalpy of dissolution can have both positive and negative values. So the dissolution of the crystalline potassium hydroxide accompanied by the release of heat
Δ H solution KOH o \u003d Δ H resh o + Δ H hydrK + o + Δ H hydroOH −o = −59 kJ/mol
Under the enthalpy of hydration - Δ H hydr, refers to the heat that is released during the transition of 1 mole of ions from vacuum to solution.
Standard enthalpy of neutralization
Standard enthalpy of neutralization - Δ H neutral about the enthalpy of the reaction of interaction of strong acids and bases with the formation of 1 mole of water under standard conditions:
HCl + NaOH = NaCl + H2O
H + + OH - \u003d H 2 O, ΔH neutral ° \u003d -55.9 kJ / mol
Standard enthalpy of neutralization for concentrated solutions strong electrolytes depends on the concentration of ions, due to the change in the value of ΔH hydration ° ions when diluted.
Enthalpy
Enthalpy is a property of matter that indicates the amount of energy that can be converted into heat.
Enthalpy is a thermodynamic property of a substance that indicates the level of energy stored in its molecular structure. This means that while matter can have energy based on temperature and pressure, not all of it can be converted to heat. Part of the internal energy always remains in the substance and maintains its molecular structure. Part kinetic energy substance is not available when its temperature approaches the ambient temperature. Therefore, enthalpy is the amount of energy that is available for conversion into heat at a certain temperature and pressure. Enthalpy units- British thermal unit or joule for energy and Btu/lbm or J/kg for specific energy.
Enthalpy amount
Quantity enthalpy substance based on its given temperature. Given temperature is the value chosen by scientists and engineers as the basis for calculations. This is the temperature at which the enthalpy of a substance is zero J. In other words, the substance has no available energy that can be converted into heat. This temperature at various substances different. For example, this temperature of water is the triple point (0°C), nitrogen is -150°C, and refrigerants based on methane and ethane are -40°C.
If the temperature of a substance is above its given temperature, or changes state to gaseous at a given temperature, the enthalpy is expressed as a positive number. Conversely, at a temperature below a given enthalpy of a substance is expressed as a negative number. Enthalpy is used in calculations to determine the difference in energy levels between two states. This is necessary to set up the equipment and determine coefficient usefulness of the process.
Enthalpy is often defined as the total energy of matter, since it is equal to the sum of its internal energy (u) in a given state, along with its ability to do work (pv). But in reality, enthalpy does not indicate full energy substances at a given temperature above absolute zero (-273°C). Therefore, instead of defining enthalpy as the total heat of a substance, it is more accurate to define it as the total amount of available energy of a substance that can be converted to heat. H=U+pV
Internal energy
The internal energy of a body (denoted as E or U) is the sum of the energies of molecular interactions and thermal motions of a molecule. The internal energy is a single-valued function of the state of the system. This means that whenever the system is in a given state, its internal energy takes on the value inherent in this state, regardless of the history of the system. Consequently, the change in internal energy during the transition from one state to another will always be equal to the difference between its values in the final and initial states, regardless of the path along which the transition was made.
The internal energy of a body cannot be measured directly. Only the change in internal energy can be determined:
Attached to the body heat, measured in joules
- Work, performed by the body against external forces, measured in joules
This formula is a mathematical expression first law of thermodynamics
For quasi-static processes the following relation holds:
-temperature, measured in kelvins
-entropy, measured in joules/kelvin
-pressure, measured in Pascals
-chemical potential
Number of particles in the system
Ideal gases
According to Joule's law, derived empirically, the internal energy ideal gas independent of pressure or volume. Based on this fact, we can obtain an expression for the change in internal energy ideal gas. By definition molar heat capacity at a constant volume 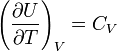 . Since the internal energy of an ideal gas is a function of temperature only, then
. Since the internal energy of an ideal gas is a function of temperature only, then
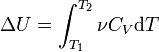 .
.
The same formula is also true for calculating the change in the internal energy of any body, but only in processes with a constant volume ( isochoric processes); in general C V (T,V) is a function of both temperature and volume.
If we neglect the change in molar heat capacity with a change in temperature, we get:
Δ U = ν C V Δ T,
where ν is the amount of substance, Δ T- temperature change.
INTERNAL ENERGY OF A SUBSTANCE, BODY, SYSTEM
(Greek: ένέργια - activity, energy). Internal energy is part total body energy (systems tel): E = E k + E p + U, where E k - kinetic energy macroscopic movements systems, E p - potential energy, due to the presence of external force fields(gravitational, electric, etc.), U- internal energy. Internal energy substances, bodies, systems of bodies - function states, defined as the total energy reserve of the internal state of a substance, body, system, changing (released) in process chemical reactions, heat transfer and performance work. Components of internal energy: (a) kinetic energy of thermal probabilistic movement of particles (atoms, molecules, ions etc.), constituting a substance (body, system); (b) potential energy of particles due to their intermolecular interaction; (c) energy of electrons in electron shells, atoms and ions; (d) intranuclear energy. Internal energy is not related to the process of changing the state of the system. With any changes in the system, the internal energy of the system, together with its environment, remains constant. That is, internal energy is neither lost nor gained. At the same time, energy can move from one part of the system to another or be transformed from one forms to another. This is one of the expressions law conservation of energy - the first law of thermodynamics. Part of the internal energy can be converted into work. This part of the internal energy is called free energy - G. (In chemical compounds it is called chemical potential). The rest of the internal energy, which cannot be converted into work, is called bound energy - W b .
Entropy
Entropy (from Greekἐντροπία - turn, transformation) into natural sciences- measure of disorder systems, consisting of many elements. In particular, in statistical physics - measure probabilities realization of any macroscopic state; in information theory- a measure of the uncertainty of any experience (test), which can have different outcomes, and hence the number information; in historical science, for explications phenomenon alternative history (invariance and variability historical process).
After proving the existence of atoms and molecules major discovery atomic-molecular theory was the law of conservation of mass, which was formulated in the form of a philosophical concept by the great Russian scientist Mikhail Vasilyevich Lomonosov (1711-1765) in 1748 and confirmed experimentally by himself in 1756 and independently by the French chemist A.L. Lavoisier in 1789
The mass of all substances entering into a chemical reaction is equal to the mass of all products of the reaction.
Experiments on the combustion of substances, which were carried out before Lomonosov, suggested that the mass of substances in the reaction process is not conserved. When heated in air, mercury turned into red scale, the mass of which was greater than that of the metal. The mass of ash formed during the combustion of wood, on the contrary, is always less than the mass of the original substance.
Lomonosov conducted a simple experiment, which showed that the combustion of a metal is an addition reaction, and an increase in the mass of the metal occurs due to the addition of part of the air. He calcined metals in a sealed glass vessel and found that the mass of the vessel did not change, although a chemical reaction was taking place. After the vessel was opened, air rushed into it, and the mass of the vessel increased. Thus, with an accurate measurement of the mass of all participants in the reaction, it turns out that the mass of substances in a chemical reaction is preserved. The law of conservation of mass was of great importance for atomic and molecular theory. He confirmed that atoms are indivisible and do not change during chemical reactions. Molecules exchange atoms during the reaction, but total number atoms of each type does not change, and therefore the total mass of substances in the reaction process is preserved.
The law of conservation of mass is a special case of a general law of nature - the law of conservation of energy, which states that the energy of an isolated system is constant. Energy is a measure of movement and interaction various kinds matter. For any process in isolated system energy is neither produced nor destroyed, it can only change from one form to another.
One form of energy is the so-called rest energy, which is related to mass by the Einstein relation
E 0 \u003d m 0 s 2,
where c is the speed of light in vacuum (c = 3 10 8 m/s). This relationship shows that mass can be converted into energy and vice versa. This is exactly what happens in all nuclear reactions, and therefore the law of conservation of mass in nuclear processes is violated. However, the law of conservation of energy remains valid in this case, if we take into account the rest energy.
In chemical reactions, the change in mass caused by the release or absorption of energy is very small. The typical thermal effect of a chemical reaction is, in order of magnitude, 100 kJ/mol. Let's calculate how the mass changes in this case:
∆m = ∆E/s 2 = 10 5 / (3 10 8) 2 ~ 10 -12 kg/mol = 10 -9 g/mol.
Topic: Initial chemical ideas
Lesson: The essence of a chemical reaction. The law of conservation of mass of substances
The Question of Essence chemical transformation remained a mystery to natural scientists for a long time. Only with the development of atomic-molecular theory it became possible to assume how chemical reactions occur at the level of atoms and molecules.
In accordance with the atomic-molecular theory, substances are made up of molecules, and molecules are made up of atoms. In the course of a chemical reaction, the atoms that make up the original substances do not disappear and new atoms do not appear.
Then, we can assume that as a result of a chemical reaction, reaction products are formed from atoms that were previously part of the original substances. Here is a model of a chemical reaction:
Rice. 1. Model of a chemical reaction from the standpoint of AMT
After analyzing this model, we can put forward a hypothesis (scientifically based assumption):
The total mass of the reaction products must be equal to the total mass of the starting materials.
Even Leonardo da Vinci said: "Knowledge, not verified by experience, the mother of all certainty, is fruitless and full of errors." This means that a hypothesis will never become a law unless it is confirmed experimentally.
The experimental method in chemistry began to be widely used after the research of R. Boyle in the 17th century. The English naturalist calcined metals in unsealed vessels - retorts and found that after calcination, the mass of the metal became larger.
Based on these experiments, he did not take into account the role of air and made the wrong conclusion that the mass of substances changes during chemical reactions.
M.V. Lomonosov, unlike R. Boyle, calcined metals not in the open air, but in sealed retorts and weighed them before and after calcination. He proved that the mass of substances before and after the reaction remains unchanged and that when calcined, air is added to the metal (oxygen had not yet been discovered at that time). But Lomonosov did not publish the results of his research.
In 1774, R. Boyle's experiments were repeated by A. Lavoisier with exactly the same results as Lomonosov. But he made a new, very important observation, namely, that only a part of the air in the sealed retort was combined with the metal, and that the increase in the weight of the metal that has passed into the scale is equal to the decrease in the weight of the air in the retort. However, part of the metal remained in free form.
Thus, independently of each other, M.V. Lomonosov and A. Lavoisier confirmed the validity of the assumption about the conservation of the mass of substances as a result of a chemical reaction.
This assumption became law only after a ten-year study by the German chemist G. Landolt at the beginning of the 20th century. Today law of conservation of mass is formulated like this:
The mass of substances participating in the reaction is equal to the mass of the reaction products.
The correctness of the law of conservation of mass of substances can be confirmed using the following experiment. In the first Landolt vessel, prepare solutions of potassium iodide and lead nitrate. In the second vessel, the reaction of ferric chloride with potassium thiocyanate will take place. Close the plugs tightly. We balance the scales. Will the equilibrium be maintained after the end of the reactions? In the first vessel, a yellow precipitate of lead iodide precipitates; in the second, dark red ferric thiocyanate is formed. Chemical reactions took place in Landolt's vessels: new substances were formed. But the balance was not disturbed (Fig. 2). The mass of the starting materials is always equal to the mass of the reaction products.

Rice. 2. An experiment confirming the correctness of the law of conservation of mass of substances
Let us give an example of another experiment proving the correctness of the law of conservation of the mass of substances in chemical reactions. Inside the flask, when the cork is closed, a candle will burn. Let's balance the scales. Let's set fire to the candle and lower it into the flask. Close the flask tightly with a stopper. The burning of a candle is chemical process. Having used up the oxygen in the flask, the candle goes out, the chemical reaction is completed. But the balance of the weights is not disturbed: the mass of the reaction products remains the same as the mass of the initial substances (Fig. 3).

Rice. 3. Experiment with a burning candle in a flask
The discovery of the law of conservation of mass of substances was of great importance for the further development of chemistry. Based on the law of conservation of mass of substances, the most important calculations are made and equations of chemical reactions are compiled.
1. Collection of tasks and exercises in chemistry: 8th grade: to the textbook by P.A. Orzhekovsky and others. "Chemistry, Grade 8" / P.A. Orzhekovsky, N.A. Titov, F.F. Hegel. – M.: AST: Astrel, 2006.
2. Ushakova O.V. Chemistry workbook: 8th grade: to the textbook by P.A. Orzhekovsky and others. “Chemistry. Grade 8” / O.V. Ushakova, P.I. Bespalov, P.A. Orzhekovsky; under. ed. prof. P.A. Orzhekovsky - M .: AST: Astrel: Profizdat, 2006. (p. 15-16)
3. Chemistry: 8th grade: textbook. for general institutions / P.A. Orzhekovsky, L.M. Meshcheryakova, L.S. Pontak. M.: AST: Astrel, 2005.(§6)
4. Chemistry: inorg. chemistry: textbook. for 8 cells. general institutions / G.E. Rudzitis, FuGyu Feldman. - M .: Education, JSC "Moscow textbooks", 2009.
5. Encyclopedia for children. Volume 17. Chemistry / Chapter. edited by V.A. Volodin, leading. scientific ed. I. Leenson. – M.: Avanta+, 2003.
Additional web resources
1. A single collection of digital educational resources ().
2. Electronic version of the journal "Chemistry and Life" ().
Homework
With . 16 №№ 3,5 from Workbook in chemistry: 8th grade: to the textbook by P.A. Orzhekovsky and others. “Chemistry. Grade 8” / O.V. Ushakova, P.I. Bespalov, P.A. Orzhekovsky; under. ed. prof. P.A. Orzhekovsky - M.: AST: Astrel: Profizdat, 2006.
1. The law of conservation of mass and energy.
This is a combined law. It includes two laws.
I. Law of conservation of mass : The mass of substances involved in the reaction is equal to the mass of the reaction products.
This law was discovered by M. V. Lomonosov in 1748 and supplemented by A. L. Lavoisier in 1789.
During the reaction, the mass of each 1 element.
This law allows you to make equations of chemical reactions and carry out calculations based on them. It is not absolute (see below). The law of conservation of energy is absolute.
2. The law of conservation of energy: Energy does not arise from nothing and does not disappear, but only passes from one form to another.
This law is the result of the work of A. Einstein. He established the connection between energy and the mass of matter (1905):
E \u003d ts 2,(6)
where With- the speed of light in vacuum, equal to -300,000 km/s. Since energy is released or absorbed as a result of a chemical reaction, then, in accordance with the Einstein equation, the mass of substances also changes. However, this change is so small that it is not taken into account in practice (the so-called mass defect).
The formation of one mole of hydrogen chloride from simple substances is accompanied by a thermal effect of 92.3 kJ / mol, which corresponds to a loss of mass of the substance (“mass defect”) of about 10 -9 g.
The following laws are valid only for compounds with a constant molecular composition- daltonids. They differ from compounds having a variable composition of molecules - berthollids.
Metal alloys contain compounds of the type M t M l, where t and n- variables.
2. Law of constancy of composition (J. L. Proust, 1801).
The ratio between the masses chemical elements, which are part of this compound, is a constant value, independent of the method of its preparation.
3. Law of multiple ratios (J. Dalton, 1803).
If two elements form several chemical compounds, then the masses of one of the elements per a certain mass of the other are related to each other as small integers.
In carbon monoxide (II) CO: M (C) / M (O) \u003d 12/16 \u003d 3/4, in carbon monoxide (IV) CO 2: M (C) / M (2 O) \u003d 12/32 \u003d 3 /eight. Therefore, the masses of carbon per a certain mass of oxygen in these compounds are related as:
3 / 4: 3 / 8 =2:1
4. Law of simple volumetric ratios (J. L. Gay-Lussac, 1808).
The volumes of reacted gases are related to each other and to the volumes of gases formed as small integers.
In the reaction of ammonia formation in accordance with the stoichiometric coefficients in the reaction equation:
H 2 + 3N 2 = 2NH 3 we get that V (N 2) : V (H 2) : V (NH 3) = 1:3:2.
5. Law of Avogadro (1811). Equal volumes of different gases under the same conditions (p and T) contain the same number of molecules.
This law follows from the analysis of the Mendeleev-Clapeyron ideal gas equation of state:
pV = nRT.
This equation can be written for two gases: p 1 V 1= V 1 RT 1, p 2 V 2= V 2 RT 2 .
If equal p 1 \u003d p 2, T 1 \u003d T 2 and V i = V 2 the amounts of substances of gases will be equal: n 1= n 2 or, given the Avogadro number:
n 1 N A \u003d n 2 N A,
i.e., the number of molecules of these gases will also be equal.
Avogadro's law has consequences:
1. The same number of molecules of any gas under the same conditions occupies the same volume.
2. The masses of gases taken in equal volumes under the same conditions (p, T) are related to each other as their molar masses:
t 1 / t 2 \u003d M 1 / M 2.(7)
This consequence follows from the equality of the amounts of substances of these gases (see above): ν 1 = ν 2 .
Substituting instead of the amount of a substance the ratio of its mass to the molar mass (equation 2) we get:
t 1 / M 1 \u003d t 2 / M 2
t 1 / t 2 \u003d M g / M 2.
The second corollary allows us to derive an equation for determining the molar mass of an unknown gas from a known value of the relative density of this gas from another known gas.
After substituting into the numerator and denominator of the left side of the equation 7 volumes of the first and second gases, which are equal, we get:
t 1· V 2 / t 2 V 1 \u003d M 1 / M 2.
The ratio of the mass of a substance to its volume is replaced by density (see equation 5):
R 1 / R 2 \u003d M 1 / M 2
and we obtain an equation for calculating the molecular weight of the first gas from the second:
M 1= (ρ 1 / ρ 2) M 2 = D 1/2 M 2(8)
Or in general view:
M=D G M g(9)
where D G- relative density of the first gas in relation to the second.
If the hydrogen density of a given gas is known, then the following equation is used:
M \u003d 2DH 2.(10)
If the density of the gas in air is known, then the equation is used:
M = 29D air. (11)