Energy levels of atoms. Bohr postulates and orbit quantization
For an electron under the action of forces of attraction to the nucleus, the Schrödinger equation has solutions not for any, but only for certain values of energy. Thus, the quantization of the energy states of an electron in an atom (ie, Bohr's first postulate) turns out to be a consequence of the wave properties inherent in the electron and does not require the introduction of special postulates.
And when something is not good for you, the muscle actually shows weakness, but only because the energy that passes through it is weakened. Your energy is weakened when you have negative thoughts about yourself or others, but also when a substance, food or medicine, intention or decision is not right for you or simply not in your best interest.
What is an energy test?
This is an honest reaction of your energy, "favorable" to the reaction of the muscles in your body. Raise your hand up and down as shown in the picture, extend your hand, but do not strain too much, and say your name. He probably won't be able to move his arm as the muscle will remain strong because your energy is consistent with the statement and knows it's true for you.
For a better understanding of the last statement, consider a simplified model of the atom, a "one-dimensional atom", in which an electron can only make oscillatory movements between extreme points.
We will also assume that the boundaries of the atom are impenetrable for the electron, so that it can only be inside the atom. We already know that the state of an electron in an atom is characterized by a certain wave (“de Broglie wave”). But it would be wrong to imagine the propagation of this wave as something similar to the movement of a wave formed on the surface of water from a thrown stone: a water wave moves indefinitely from the place of its formation and gradually spreads out, it does not have stability in time, while an electron in an atom is stable. Therefore, the analogy between the state of an electron in an atom and the state of a sounding string on which so-called standing waves are formed will be more correct.
Let your friend test the strength of your hand and see how easily it will take it off. Your energy system knows this is not true. Now imagine something pleasant and joyful, and the test will show the strength of the muscles, and your arm will remain extended. Consider a negative thought or something bad for the other person or imagine crying and being sad and your hand will be easily removed. You will see how it weakens your energy when you are negative, you are under stress or something is wrong or not good for you.
Of course, it's simple and general description energy test. He could tell you many things, and if you are very amateur, you deal with him. However, when used as a tool in energy psychology, it becomes an extremely reliable and safe means of detecting energy meridian blockages, as well as a means of finding the most appropriate way to balance and restore energy flow and therefore your health.
On fig. 6 schematically depicts standing waves arising on an oscillating string, the extreme points of which are fixed. Antinodes appear at the points marked with the letter n - here the oscillation amplitude is maximum, at the points y the string does not oscillate - these are nodes at which the oscillation amplitude is zero; at points located between nodes and antinodes, the oscillation amplitude has intermediate values. Because the endpoints of the string are fixed, there are bound to be knots here. Unlike an ordinary "traveling" wave, a standing wave does not move in space and does not transfer energy, which is only transferred from one point of the string to another. It is easy to see (Fig. 6) that on a string with fixed ends, the length of the standing wave may not be any, but only such that the whole string fits integer half-waves: one (Fig. 6, a), two (Fig. 6, b), three (Fig. 6, c), etc.
The energy testing methods described above are done with a partner, who can be your therapist or a friend. There are other ways to test yourself and get the answers you need. They are not difficult, affordable and easy to digest. This is another way to take charge of your own health and learn to "speak" to your own energy.
This way, you can reliably test the decisions you have doubts about, the purely life decisions you face, and many other things that are important to your health and life. The possibilities of the energy test are practically unlimited, simply because it is a shortcut to your subconscious mind where all the answers really are.
In the one-dimensional model of the atom under consideration, the de Broglie wave must also be standing: this follows from the fact that the electron cannot go beyond the boundaries of the atom and, therefore, the wave function (i.e., the amplitude of the wave) must vanish at the boundaries of the atom. Therefore, fig. 6 can be considered as a model of a one-dimensional atom with de Broglie standing waves that can form in this atom.
If the length of a one-dimensional atom is equal to l, then for cases a, b, and c in Figs. 6 the de Broglie wavelength will be expressed as follows:
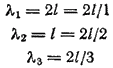
Therefore, a standing wave can form only if
where , i.e. an integer.
Rice. 6. Standing waves on a string.
On the other hand, according to the de Broglie equation
Equating the right parts of the last two equations, we obtain the expression for the electron velocity:
Now, knowing the speed of the electron, we can find it kinetic energy E:
Since n is an integer, the last expression shows that the energy of an electron in a one-dimensional atom cannot have arbitrary values: for n=1 it is equal to the value of the fraction, for n=2 it is 4 times greater, for n=3 - 9 times more, etc. Thus, in the case of a one-dimensional atom wave properties electron, expressed by the de Broglie equation, indeed have as a consequence the quantization of the energy states of the electron. In this case, the allowable energy levels of an electron are determined by the value of an integer n, called the quantum number.
Of course, the found expression for the electron energy refers to a simplified model of the atom. But for a real atom, the solution of the Schrödinger equation also leads to the conclusion that the energy states of an electron in an atom are quantized.
The model of a one-dimensional atom makes it possible to understand why an electron in an atom in a stationary state does not radiate electromagnetic energy (the second postulate of Bohr's theory). According to the Bohr-Rutherford model, an electron in an atom made continuous motion with acceleration, that is, it changed its state all the time; in accordance with the requirements of electrodynamics, it must radiate energy at the same time. In the one-dimensional model of the atom, the stationary state is characterized by the formation of a standing de Broglie wave; as long as the wavelength of this wave remains constant, the state of the electron remains unchanged, so that no radiation should occur.
The question of the state of the electron during the transition from one stationary state to another (in Bohr's terminology, from one stationary orbit to another) also becomes clear. If, for example, an electron from the state corresponding to Fig. 6, a, goes into the state corresponding to Fig. 6b, then during this transition the de Broglie wavelength will have a variable value that does not meet the condition for the formation of a standing wave. That is why the state of the electron in this period of time will be unstable; it will change until the de Broglie wavelength again corresponds to the condition for the formation of a standing wave, that is, until the electron is in a new stationary state.
In a simplified one-dimensional model of an atom, the position of an electron relative to the nucleus is determined by one coordinate, and its state is determined by the value of one quantum number. In a two-dimensional (flat) model of an atom, the position of an electron is determined by two coordinates; in accordance with this, its state is characterized by the values of two quantum numbers. Similarly, in a three-dimensional (volume) model of an atom, the state of an electron is determined by the values of three quantum numbers. Finally, the study of the properties of electrons that make up real atoms showed that the electron has one more quantized physical characteristic (the so-called spin, see § 30), which is not related to the spatial position of the electron. Thus, for a complete description of the state of an electron in a real atom, it is necessary to indicate the values of four quantum numbers.
28. Main quantum number. So, in a one-dimensional model of an atom, the energy of an electron can only take certain values, in other words, it is quantized. The energy of an electron in a real atom is also a quantized quantity. The possible energy states of an electron in an atom are determined by the value of the main quantum number n, which can take positive integer values: 1, 2, ... etc. The electron has the lowest energy at n = 1 with an increase in n, the energy of the electron increases. Therefore, the state of an electron, characterized by a certain value of the main quantum number, is usually called the energy level of an electron in an atom: at n=1, the electron is at the first energy level, at n=2 - at the second, etc.
The main quantum number also determines the size of the electron cloud. In order to increase the size of the electron cloud, it is necessary to move part of it to a greater distance from the nucleus. This is prevented by the forces of electrostatic attraction of the electron to the nucleus, the overcoming of which requires the expenditure of energy. That's why large sizes The electron cloud corresponds to a higher energy of an electron in an atom and, consequently, a larger value of the principal quantum number n. The electrons, which are characterized by the same value of the main quantum number, form electron clouds of approximately the same size in the atom; Therefore, we can talk about the existence of electron layers in the atom or electron shells corresponding to certain values of the principal quantum number.
For the energy levels of an electron in an atom (i.e., for electron layers, or shells) corresponding to different values of n, the following letter designations are accepted.
The state of an electron in an atom is characterized by four quantum numbers. The main quantum number n determines the energy of an electron in an atom and the size of the AO, i.e. distance of the electron from the nucleus. The main quantum number n takes on the values of integers 1, 2, 3, 4… The set of electrons with the same value of n is called the energy level. The electrons of the first energy level from the nucleus (n=1) have the lowest energy; as n increases, the energy of the electron and its distance from the nucleus increase. The state of an atom, when its electrons are at such energy levels that their total energy is minimal, is called the main or unexcited state. States with higher energy values are called excited states. Energy levels are denoted by letters:
Numeric value n 1 2 3 4 5 6 7
Letter designation K L M N O P Q
The number of energy levels in an atom in the ground state is equal to the number of the period in which the element is located.
At the same energy level there can be atomic orbitals of various shapes, differing from each other in energy. Therefore, the energy levels are divided into sublevels. The electron energy at the sublevel and the shape of the atomic orbital are characterized by the orbital quantum number l. Meaning l depends on the principal quantum number: l takes values from 0 to (n–1), i.e. 0, 1, 2, 3… (n–1). Within a given energy level, a set of electrons characterized by the same value l, is called the energy sublevel. Sublevels are denoted by letters:
Orbital quantum number l 0 1 2 3
Energy sublevel designation s p d f
Thus, at l = 0, 1, 2, 3 the electrons are respectively on the s-, p-, d-, f-sublevels. Electrons of various sublevels are called s-, p-, d-, f-electrons. In this case, one also speaks of the states of s-, p-, d-, f-electrons or s-, p-, d-, f-atomic orbitals.
18. Principal quantum number.
The principal quantum number is an integer that is the definition of the state of an electron on an energy level. Energy level is a set of stationary states of an electron in an atom with close energy values. The principal quantum number determines the distance of the electron from the nucleus, and characterizes electron energy that occupy this level.
19. Orbital quantum number. Forms of electron clouds
Orbital quantum number- in quantum physics, the quantum number ℓ, which determines the shape of the distribution of the amplitude of the wave function of an electron in an atom, that is, the shape of the electron cloud. Determines the sublevel of the energy level specified by the main (radial) quantum number n and can take the values
Is an eigenvalue operator orbital momentum electron, which differs from the angular momentum of the electron j only on the spin operator s:
The difference between the orbital quantum number and the quantum number of the total momentum does not exceed, in absolute value, (electron spin). The azimuthal quantum number determines the orientation of the electron cloud in space.
According to the mechanistic model electron cloud shape atom is a consequence of the shape of the nucleus of the atom. The sources of forces that bind atoms to each other are the overlap zones of electron clouds. The overlap zones are simultaneously part of the electron clouds of both contacting atoms. The more overlap zones, the stronger the bond between atoms. Each electron of the electron cloud on most of its trajectory interacts mainly with one of the protons of the nucleus, forming part of the electron cloud - an electron lobe. But in overlap zones, electrons can move from one electron lobe to another. The electron petals associated with the protons of the completed nuclear shell do not participate in interatomic bonds. They merge into a continuous electron cloud, the boundaries of which are much closer to the center of the nucleus than the ends of isolated electron petals. For this reason, a continuous electron cloud does not reach the electron clouds of neighboring atoms and is not capable of creating overlap zones with them. Interatomic bonds are capable of creating only isolated electron petals. They stretch far beyond the solid electron clouds and are attracted to similar electron lobes of other atoms, creating overlap zones. The attraction of atoms occurs until a balance is reached between the gravidynamic forces of attraction and the forces of elasticity of the electron shell. It follows from this that the boundaries of the electron cloud of an atom are not rigidly fixed and can change with a change in the density of atoms. Accordingly, the size of the atom can also change. Let's try to construct theoretically possible configurations of overlap zones between electron clouds of identical atomic isomers at maximum packing. Such stuck together atoms of the same type are capable of forming a single crystal - a bulky giant molecule with a periodic structure.






