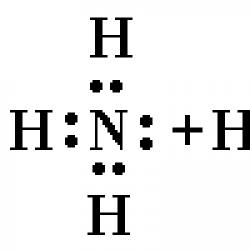Chemical bond in ammonium chloride. Types of chemical bonds. Chemical properties of ammonium chloride
§5. And for salts, the traditional one is an ionic bond
What type of chemical bond will it have if it is formed by atoms of elements that are very different in electronegativity - for example, an alkali metal sodium and halogen chlorine?
Atom electron sodium Na, located on the outer valence level, is a big fan of walking around the neighborhood and often even forgets to return home to his native atomic nucleus. And then the sodium atom is left without its wayward electron and turns into cation Na+:
Na 0 − 1 e− = Na +
Seven electrons of an atom chlorine Cl On the contrary, they are incorrigible homebodies; they do not like to visit, but they gladly receive guests. If some neighboring electron turns towards them, it means the atom chlorine will turn into anion Cl¯, which is usually called chloride-ion:
Cl 0 + 1 e− = Cl −
Ions of opposite charge will attract each other; it is formed Na+ ionic bond ~~~~ Cl −. So it turns out sodium chloride NaCl, everyone knows salt.
Natural table salt is a mineral.
In nature, this mineral is formed from salty sea or lake water. When it is cooled under a layer of salt solution, its crystals are discovered - transparent cubes sodium chloride. The ions that make up sodium chloride, form ionic crystal, consisting of cations sodium Na+ And chloride anions Cl −.
When a solid is formed from dissolved ions, the cations and anions are arranged in the crystal not haphazardly, but in such a way that positive and negative charges alternate with each other. Then it will be strong crystal cell salt NaCl, consisting of ions.
Crystals of other salts are built in a similar way - sodium carbonate Na 2 CO 3 (soda), ammonium chloride NH4Cl (ammonia), silver nitrate AgNO3 (lapis) and many others.
Is it true that all salts are built from individual ions that exist alone? True, but there is a small “but”...
The salt cation retains some portion of the electron cloud, because even the naughtiest electron returns home from time to time. But a very large part of this cloud is shifted towards the salt anion. Therefore, the ionic bond is considered limiting case of covalent polar bond.
DEFINITION
Ammonium– a positively charged polyatomic ion.
Chemical formula NH4+
The ammonium ion NH 4 + is a regular tetrahedron with at the center and atoms at the vertices of the tetrahedron.
In the ammonia molecule NH 3 three electron pairs form three N – H bonds, the fourth electron pair belonging to the nitrogen atom is lone. With the help of this electron pair, a bond is formed with a hydrogen ion, which has an empty orbital:

Thus, in the ammonium ion, three covalent bonds are formed by the exchange mechanism, and one by the donor-acceptor mechanism. The formation mechanism does not affect the characteristics of the bond; all bonds in the ammonium cation are equivalent.
Ammonium compounds
The ammonium cation can form ammonium compounds with various counterions, in which a positively charged nitrogen atom is covalently bonded to hydrogen ions and (or) organic radicals, and ionically bonded to some anion.
Inorganic ammonium compounds
Ammonia hydrate(ammonium hydroxide, ammonia water, ammonium hydroxide, ammonia hydroxide). Formula: NH 3 H 2 O
Formed when ammonia reacts with water. A weak base dissociates in water to form ammonium cations and hydroxide ions:
The reaction is reversible, therefore aqueous solutions of ammonium hydroxide always have a characteristic pungent odor of ammonia.
Ammonium salts
All ammonium salts are similar in properties to the corresponding sodium salts. They dissolve well in water, completely dissociate in an aqueous solution, and decompose when heated:
In solution they hydrolyze by cation:
Organic ammonium compounds divided by the number of organic radicals associated with the nitrogen atom into primary (R 1 NH 3) + X –, secondary (R 1 R 2 NH 2) + X –, tertiary (R 1 R 2 R 3 NH) + X –, and quaternary (R 1 R 2 R 3 R 4 N) + X – .
Primary, secondary and tertiary ammonium compounds can be considered as salts of the corresponding amines; they can be prepared by reacting these amines with acids:
where R 1 , R 2 , R 3 are organic radicals or hydrogen, X is the anion of the acid residue.
Qualitative reaction for ammonium ions - interaction with alkalis with the release of ammonia:
Examples of problem solving
EXAMPLE 1
| Exercise | What pH value (more or less than 7) does an aqueous solution of ammonium chloride have? Write down the molecular and ion-molecular equations of hydrolysis. |
| Solution | NH 4 Cl is a salt of a weak base and a strong acid, so hydrolysis occurs through the cation. Molecular equation: Full ionic equation: Brief ionic equation: During the hydrolysis process, hydrogen (H +) was formed, so the solution has an acidic environment (pH |
| Answer | The pH of the ammonium chloride solution is less than 7. |
EXAMPLE 2
| Exercise | What mass of salt is formed by the interaction of 44.8 liters of ammonia and 33.6 liters of hydrogen chloride (normal conditions)? | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solution | Let's write the reaction equation: The molar masses of ammonia, hydrogen chloride and the resulting salt, ammonium chloride (NH 4 Cl), are equal. Chemical bond. Crystal cell The answers to the tasks are a word, phrase, number or sequence of words, numbers. Write your answer without spaces, commas or other additional characters. Beginning of the form 1 Ammonium chloride contains chemical bonds: 1. ionic 2. covalent polar 3. covalent nonpolar 4. Hydrogen 5. metal 2 Intermolecular hydrogen bonds in the liquid state are characteristic of: 1. hydrogen 2. Water 3. Ammonia 4. Acetaldehyde 5. isobutane 3 Covalent nonpolar chemical bonds are found in substances: 1. white phosphorus 2. phosphoric acid 3. ammonia 4. ethyl alcohol 5. rhombic sulfur 4 From the list provided, select two compounds that contain an ionic chemical bond. 1. Ca(ClO 2 ) 2 2. HClO 3 3. NH 4 Cl 4.HClO 4 5. C l2O 7 5 From the list provided, select two compounds that contain covalent nonpolar chemical bond. 1. Ca 2. N 2 3. AlCl 3 4. HClO 4 5. Cl 2 6 Potassium sulfate contains chemical bonds: 1. ionic 2. covalent nonpolar 3. covalent polar 4. hydrogen 5. metal 7 Both ionic and covalent chemical bonds are present in the substance: 1. HCl 2. H 2 SO 4 3. NaOH 4. NH 4 Br 5. C 2 N 5 HE 8 From the proposed list, select two compounds between the molecules of which a hydrogen bond. 1. phenol 2. diethyl ether 3. Ethyl acetate 4. formic aldehyde 5. formic acid 9 From the list provided, select two compounds that contain a covalent polar chemical bond. 1. CaCl 2 2. HCl 3. BaO 4. KSIO 4 5. Cl 2 10 They have a non-molecular structure: 1. potassium hydroxide 2. Ammonia 3. acetic acid 4. nitric acid 5. graphite 11 Ionic bonds are realized in each of two substances: 1. Al 2 O 3 AndFeCl 3 2.K 2 SAndNaNO 3 3. KNO 2 and NO 2 4. HF and HCl 5. NaBr and NH 4 F 12 The atomic crystal lattice in the solid state has: 1. oxygen 2. white phosphorus 3. red phosphorus 4. diamond 5. sodium chloride 13 From the list provided, select two compounds in which a chemical bond is formed due to a shared pair of electrons. 1. Ca 2. H 2 O 3. NaCl 4. CaO 5. Cl 2 14 The molecular structure is: 1. propanol-2 2. potassium acetate 3. carbon dioxide 4. sodium methoxide 5. calcium carbonate 15 All substances with ionic crystal lattices 1. hard 2. plastic 3. relatively volatile 4. highly soluble in water 5. have high melting points 16 From the list provided, select two compounds whose molecules form a hydrogen bond. 1. methane 2. Silane 3. Ammonia 4. Phosphine 5. Water End of form Ammonium chloride (ammonium chloride, ammonia) is an inorganic compound widely used in a variety of fields. From a chemical point of view, it is an ammonium salt; formula NH 4 Cl. Ammonium chloride was already known in Ancient Greece and Ancient Egypt. One of its names, “ammonia” comes from the Egyptian “nushadir” - a substance that priests collected on the walls of caves in order to inhale its vapors before the ceremony and tune in to communicate with divine forces. "Ammonia" traces its name back to the Egyptian sun god Amun. In Ancient Greece, the substance was extracted from soot that formed on the walls of chimneys as a result of the burning of camel dung, which served as fuel for the Greeks (ammonium chloride is obtained from the decomposition of animal and human excretions). Ammonium chloride occurs naturally in volcanic caves and near cracks in the earth's crust, in the form of plaque or crusts. PropertiesNH 4 Cl is a white crystalline powder (the technical grade reagent can be yellowish or pink), slightly hygroscopic, odorless, and salty in taste. It dissolves well in water and liquid ammonia; with increasing temperature, water solubility increases. Reacts with alkalis to form salt, water and ammonia. The reagent dissolves to a much lesser extent in ethyl and methyl alcohols. Burns with the release of thick white smoke. Completely decomposes at temperatures above 338 °C, as well as under the influence of electric current. Burning ammonium chloride releases ammonia, which is a respiratory irritant. An aqueous solution of the reagent is ammonia, a liquid with a pungent odor that has a stimulating effect on the nervous system. The reagent is used as a medicine, but treatment with ammonia should be carried out strictly on the recommendation of a doctor, since an overdose can lead to respiratory arrest and coma (alkaline solutions, for example, sodium bicarbonate, are administered as an antidote). In addition, this medicine has contraindications.
Ammonium chloride is classified as a moderately hazardous substance (class 3); you should work with it wearing protective equipment: rubber gloves, safety glasses and a respirator, in a room with supply and exhaust ventilation. Care must be taken to ensure that the reagent does not come into contact with the skin and mucous membranes. Store chemical The reagent is needed in hermetically sealed multilayer bags, in indoor areas without access to moisture. The connection is hazardous to the environment. ApplicationIn non-ferrous metallurgy for pickling metals. In the Prime Chemicals Group store you can buy 1. The most polar bonds in a molecule are: a) HC1 b) AsH 3 c) PH 3 r)H 2 S 2. The molecule has a linear shape: a)H 2 O b)H 2 S c)WeC1 2 d) OF 2 3. Geometric shape of the CH 4 methane molecule: a) angular b) pyramidal b) triangular d) tetrahedral 4. The molecule has a pyramidal shape: a) BC1 3 b) SiBr 4 c) A1Br 3 d) PC1 3 5. A polar molecule is: a) CO 2 b) CH 4 b) NH 3 r) N 2 6. The number of σ-bonds is three times greater than the number of π-bonds in the molecule: a) chlorous acid b) orthophosphoric acid c) sulfurous acid d) perchloric acid 7. In which series are the formulas of compounds with only covalent bonds presented? a) BaCl 2, CdC1 2, LiF c) NaCl, CuSO 4, Fe(OH) 3 b) H 2 O, SiO 2, CH3COOH d) N 2, HNO 3, NaNO 3 8. What type of chemical bonds are absent in ammonium chloride? a) covalent polar b) covalent non-polar c) donor-acceptor d) ionic 9. Chemical bond formed between atoms of elements with atomic numbers 3 and 9: a) covalent polar b) metal c) covalent nonpolar d) ionic 10. How many electrons are contained in an ethylene molecule? Not participate in the formation of chemical bonds? a) 4 b) 8 c) 12 d) 16 11. The number of electrons involved in the formation of chemical bonds is greatest in a molecule: a)H 2 O b)C1 2 b)H 2 S r)N 2 12. The atomic crystal lattice has: a) sodium hydroxide c) iron b) diamond d) ice 13. What type of crystal lattice is characteristic of compounds of s-metals with p-elements that have high electronegativity? a) metallic b) atomic b) ionic d) molecular 14. In what row are substances with atomic, molecular and ionic crystal lattices in the solid state listed, respectively? a) diamond, sodium chloride, graphite b) white phosphorus, water, chalk c) silicon (IV) oxide, copper, nitrogen d) diamond, carbon dioxide, potassium fluoride 15. What changes when ammonium chloride is formed from ammonia and hydrogen chloride? a) oxidation state of the nitrogen atom b) oxidation state and valence of the nitrogen atom c) valency of the nitrogen atom d) oxidation state of the hydrogen atom 16. Which of the following particles was formed by a donor-acceptor mechanism? a)F 2 b)HF c)BF 4 – d)BF 3 17. In which substance is the oxidation state and valence of nitrogen equal in absolute value? a)N 2 b)NH 3 b)HNO 3 d) NH 4 C1 18. Which molecule is the least stable? a)H 2 O 6)H 2 S B)H 2 Se d) H 2 Te 19. Which chemical bond is the least strong? a) metallic b) hydrogen b) ionic d) covalent 20. The atom of which element exhibits the greatest tendency to form ionic bonds? a) C b) Si c) F d) P 21. How does the polarity and bond strength change in a series of molecules HF → HC1 → HI? a) both polarity and bond strength increase b) polarity increases, strength decreases c) both polarity and bond strength decrease d) polarity decreases, strength increases 22. Which type of orbitals of hydrogen and chlorine atoms respectively overlap when forming a hydrogen chloride molecule? a) s And s b) s And R V) R And R G) p And s 23. In which molecule are all bonds polar covalent? a) H 2 O 2 b) C 2 H 4 c) C 2 H 2 d) CO 2 24. Which element has the highest algebraic value of highest oxidation state? a) fluorine b) chromium c) carbon d) chlorine 25. Which element has the lowest algebraic value of lowest oxidation state? a) nitrogen b) sulfur c) hydrogen d) bromine 26. In which compound does hydrogen have a negative oxidation state? a) NH 4 Cl b) CaH 2 c) H 2 O 2 d) HF 27. In the compounds BC1 3, Be1 2 and SiBr 4, the valence orbitals of the central atoms are respectively in the following hybrid states: a) sp, sp 2, sp 3 V) sp, sp 3, sp 2 b) sp 2 ,sp, sp 3 G) sp 3 , sp 2 , sp 28. Valency of nitrogen in the following compounds: N 2, NH 3, NH 4 +, CH 3 NH 2, HNO 3 - are equal, respectively: a) 0, III, IV, V, V c) III, III, IV, III, IV b) I, III, III, IV, IV r) III, III, III, IV, V 29.The correct characteristic of an ionic bond is: b) directional, unsaturated c) directed, saturable d) non-directional, saturable 30. The correct characteristic of a covalent bond is: a) non-directional, non-saturable b) directional, unsaturated c) directed, saturable d) non-directional, saturable 31. Double bonds between atoms exist in every compound included in the group: a) CO, CO 2 c) S 8, C 2 H 4 b) C 2 H 2, SO 2 d) CO 2, C 2 H 4 32. Triple bonds between atoms exist in each compound included in the group: a) CO, N 2 b) N 2, SO 2 c) S 8, C 2 H 2 d) CO 2, C 2 H 4 33.For which element is the highest oxidation state greater than the number of the group in which it is located in the periodic table? a) manganese b) gold c) boron d) nitrogen 34. A quantitative characteristic of elements, which allows us to judge the type of chemical bond between the atoms of these elements, is: a) atomic radius c) electronegativity b) nuclear charge d) atomic mass 35. Single polar, double nonpolar, single nonpolar, triple polar bonds exist in the molecules of the following substances, respectively: a) HF, C 2 H 4, Br 2, CO c) H 2, CO 2, HC1, N 2 b) HBr, SO 3, N 2, C 2 H 2 d) C1 2, O 2, C 2 H 6, CO 36. The total σ-number and π-bonds in a dichromic acid molecule are respectively: a) 10 and 4 b) 4 and 10 c) 6 and 2 d) 2 and 6 37. Given substances: cesium chloride, copper, diamond, rhombic sulfur, ice, sodium oxide, iodine, “dry ice” (solid CO 2), graphite, platinum, potassium hydride. Among them, the number of substances with an atomic crystal lattice is equal to: a) 4 b)3 c) 2 d)1 38. Given substances: cesium chloride, copper, diamond, rhombic sulfur, ice, sodium oxide, iodine, “dry ice” (solid CO 2), graphite, platinum, potassium hydride. Among them, the number of substances with a molecular crystal lattice is equal to: a) 4 b) 3 c) 2 d) 1 39. Given substances: cesium chloride, copper, diamond, rhombic sulfur, ice, sodium oxide, iodine, “dry ice” (solid CO 2), graphite, platinum, potassium hydride. Among them, the number of substances with an ionic crystal lattice is equal to: a) 4 b) 3 c) 2 d) 1 40. Given substances: cesium chloride, copper, diamond, rhombic sulfur, ice, sodium oxide, iodine, “dry ice” (solid CO 2), graphite, platinum, potassium hydride. Among them, the number of substances with a metal crystal lattice is equal to: a) 4 b) 3 c) 2 d) 1
|

 In industry, ammonium chloride is obtained as a by-product in the production of soda. In the laboratory, the compound can be synthesized from chlorine and ammonia, or by passing ammonia and hydrogen chloride through a solution of table salt.
In industry, ammonium chloride is obtained as a by-product in the production of soda. In the laboratory, the compound can be synthesized from chlorine and ammonia, or by passing ammonia and hydrogen chloride through a solution of table salt.