Potassium-sodium tartrate. General characteristics and preparation of potassium tartrate formula
Rochelle salt "analytical grade" (potassium-sodium tartrate, tartrate, potassium-sodium tartrate) is a tetrahydrate of sodium-potassium double salt of tartaric acid in the form of colorless or white hygroscopic crystals of the orthorhombic system. We offer to buy this product in our company.
The material is produced industrially by reacting tartaric acid with sodium and potassium carbonate salts. When producing using this method, it becomes possible to increase the size of the crystals if necessary. In laboratory practice, the reagent is synthesized by precipitation from a heated solution of potassium acid tartrate in fine-crystalline form by adding stoichiometric amounts of sodium carbonate.
Rochelle salt, which has a low price per kg, is characterized by weak alkalinity and increased hygroscopicity. It mixes well with water, but does not dissolve in ethyl alcohol. When heated to 55.6°C, the reagent begins to decompose. It has piezoelectric properties, which have led to its demand in technology: in telephone handsets, electrophone pickups, hearing aids, microphones and other similar devices.
Application area
Potassium sodium tartrate, the price of which in our company is favorable, is used as a demulsifier in aqueous solutions, as well as when carrying out reactions using aluminum hydride in organic synthesis processes. It also applies:
- as part of Feling liquid for the detection of sugars;
- for determination of proteins in solutions by the biuret method;
- as a reducing agent for silvering mirrors using the Heinrichson method;
- to increase the service life of cigarette paper in the manufacture of cigarettes;
- as an emulsifier in cheese making, antioxidant additive and leavening agent E337, as a component of baking powders in the food industry;
- as a gearbox in the printing industry;
- to increase the time interval before the plaster begins to harden in construction;
- as a component of Seydlitz powder in the production of laxatives, as well as the production of effervescent and instant preparations;
- to increase the saturation of the solution with anodes, create a good homogeneous surface and provide the ability to work at high concentrations as part of galvanic baths;
- when cleaning bronze with gilding, since the reagent does not react with copper oxides;
- as a plant growth stimulator in agriculture;
- as a desalting agent in the chemical industry.
This material is sold in our organization at an affordable price; you can buy it in 25 kg packaging.
Precautionary measures
The product is non-toxic, does not burn, does not ignite and does not form explosive mixtures with air. In terms of the degree of impact on the body, it belongs to the group of substances of the third hazard class. When working with it, you must use protective equipment: plastic goggles, thick rubber gloves, an industrial respirator, special shoes and clothing. This material is sold in rubles. at AQUAHIM LLC.
Storage Features
The reagent should be stored in closed containers in dry, ventilated warehouses. Shelf life – 36 months from the date of manufacture.
Rochelle salt: where is it sold with delivery throughout the Russian Federation?
We sell chemical products at affordable prices. If you want to buy quality goods with shipping throughout Russia, we suggest that you familiarize yourself with the full price list of our online store on the main page of this site. To place an order, simply click on the green button above and enter your contact details in the field that opens. Our employee will call you back during business hours.
Tannin E 181 is used as food coloring, for adding color, as well as in making all kinds of drinks. The dye has a strongly astringent taste. Used for coloring various baked goods and sweets. Solutions of colloids that are formed in water exhibit strong tanning effects and have an acidic reaction. Green tea contains a large amount of tannin.
Tannin E 181 is used for tanning leather and fur, for etching cotton fibers, and for making ink.
In addition, E 181 is widely used in medicine to treat diarrhea, hemorrhoids, inflammatory diseases, and to stop bleeding. You can strengthen your blood vessels by drinking green tea regularly. Also used as an antidote for poisoning with mercury, lead or other toxic substances.
In this case, the property of Tannin is used to slow down the absorption of various substances in the body. This same property results in harm from its excessive consumption - diseases associated with a lack of any minerals (for example, iron) in the body may occur. In addition, in sensitive people, this substance can disrupt the functioning of the liver and kidneys, and irritate the gastrointestinal tract.
| Technological functions | Clarifier. |
| Synonyms | Tannins, tannins, gallotannins, hydrolysable tannins, tannic acid, gallotanninic acid; English tannic acid, tanins (food grade), gallotannic acid, gallotannins; German Tannine, Tanninsaure, Gallotanninsaure, Gallotannine; fr. tannin, gallotannin, acide tanninique, acide gallotanninique. |
| CAS# | 1401-55-4. |
| Compound | Esters of phenolic acids with monosaccharides or polyhydric alcohols, for example pentamethadigalloylglucose (Chinese tannin). Catechins and other non-hydrolysable tannins, as well as ellagitannins (esters of ellagic acid) do not belong to food tannins. |
| Molecular mass | 500-3000. |
| Organoleptic properties | Amorphous powder, shiny scales or loose mass, light yellow, brownish yellow or light brown. Odorless or with a weak characteristic odor and a tart astringent taste. |
| Physicochemical characteristics | Hydrolyze to form gallic acid. Chorus. sol. in water, acetone, ethanol; Wed sol. in warm glycerin (about 1 g in 1 ml); unsolvable in benzene, chloroform, ether. |
| Natural spring | In oak and chestnut wood, in the bark of willow, larch, spruce, etc. |
| Receipt | Extraction from natural sources: 1) sumac, Sicilian or American, Rhus coriera, R. Galabra, R. Thypia; 2) ink nut, growths formed on young shoots of oak, for example. Quercus infectoria, (Chinese and Aleppo tannin); 3) seed pods of tara (Caesalpinia spinosa). Impurities: components of plant raw materials. |
| Specifications | |
| Metabolism and toxicity | They can bind bacterial toxins and toxic salts of heavy metals in the body. |
| Hygienic standards | Chipboard not specified. Allowed in the Russian Federation as consistency stabilizers, thickeners, texturizers, binding agents according to TI(clause 3.6.50 SanPiN 2.3.2.1293-03); as a dye in food products according to TI in quantities according to TI (clause 3.11.7 of SanPiN 2.3.2.1293-03); as a clarifying, filtering material, flocculant and sorbent in wines, alcoholic beverages, maximum residual amount according to TI (clause 5.1.35 of SanPiN 2.3.2.1293-03). |
| Application | Tannins have acidic properties, which are caused by phenolic groups in their molecules. Therefore, they interact with proteins, as well as with metals, precipitating them. Due to this, tannins are used to stabilize wines and beer and for processing (fining) wine materials, mainly white table wines, usually in combination with fining with gelatin. In accordance with the Instructions for processing wine materials with gelatin, approved by the Ministry of Agriculture and Food of the Russian Federation on 05.05.98, it is recommended to use 0.5-0.75 parts of tannin from the dose of gelatin used for fining, that is, 0.05-1.875 g/dal. Tannins are used in the form of a 20% solution, which is prepared on the day of processing by dissolving the required amount of tannin in the processed wine material. In case of complex fining, tannin is introduced into the wine material one day before treatment with gelatin. Tannins can be added to beer at different stages of production in amounts from 0.2 to 0.7 g/l, depending on the type of beer and the technology used. In case of overdose, instead of increasing stability, the beer becomes more cloudy; in addition, it tends to take on an empty taste. Tannins are included in the list of raw materials in GOST 28616-90 “Fruit wines. General technical conditions", GOST 28685-90 "Sparkling wines. General technical conditions". Other applications: for tanning leather, as a mordant for dyeing cotton fabrics, an astringent. |
TARTRAZINE E102
TARTRAZINE E102 is a food additive, a yellow dye. It is produced artificially, as it is not found anywhere in nature in its pure form. During production, waste from the coal industry - coal tar - is used as a raw material.
Effect on the human body:
Tartrazine is known as one of the most harmful dyes for human health. This explains the fact that the food additive E102 is prohibited in most countries. However, the food and pharmaceutical industries of Russia and Ukraine allow its use.
It has been revealed that tartrazine causes an allergic reaction in the form of urticaria. However, studies in America in 1986 showed that rashes and other adverse reactions to the dietary supplement occur no more often than once in 10,000 cases.
There is evidence that the use of E102 causes decreased concentration and excessive activity in children. It is known that, together with sodium benzoate, tartrazine can cause Merkelsson-Rosenthal syndrome, accompanied by the formation of cracks in the tongue, Quincke's edema and damage to the facial nerves.
Food additive E553 is approved in the Russian Federation in accordance with standards and technical regulations(see Hygiene Standards below).| Technological functions | Dye (monoazo dye). |
| Synonyms | English tartrazine, FD&C yellow no. 5 (USA), CI food yellow 4, acid yellow 23; German Tartrazin; fr. tartrazine, jaune tartrique. |
| CAS# | 1934-21-0 |
| Color Index - color index | 19140 |
| Chemical name | Trisodium 5-Hydroxy-1-(4-sulfonatophenyl)-4-(4-sulfonatophenylazo)-N-pyrazole-3-carboxylate. |
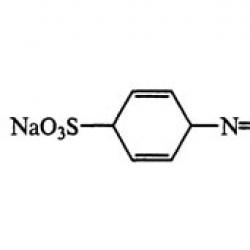
| Empirical formula | C 16 H 9 N 4 0 9 S 2 Na 3 |
| Molecular mass | 534,37 |
| Structural formula |  |
| Appearance | Powder or granulate, aqueous solution has a yellow color; aluminum varnish: powder. |
| Physicochemical characteristics | Spectrum in water: A1cm1% 426 nm (530). Sodium salt good. sol. in water; Wed sol. in ethanol; unsolvable in vegetable oils. Aluminum varnish insoluble. in water, alcohols, fats. Light fastness is good, heat resistance (up to 150°C) is very good, and resistance to acids (including fruit acids) is also good. Alkali resistance is good, but in an alkaline environment the dye gives a reddish tint. |
| Receipt | 4-Aminobenzenesulfonic acid (sulfanilic acid) is diazotized and combined with 1-(4-sulfophenyl)-5-pyrosolone-3-carboxylic acid. Impurities: sodium chloride, sodium sulfate. |
| Specifications |
 |
| Metabolism and toxicity | During the exchange process, the breakdown products of tartrazine undergo nitrogen reduction and absorption in the intestine, followed by excretion in feces and urine. In people with an allergic reaction to acetylsalicylic acid (aspirin), pseudo-allergic reactions (for example, urticaria) are occasionally possible after oral administration of tartrazine. |
| Hygienic standards | ADI 7.5 mg/kg body weight per day. In the Russian Federation it is allowed as dye in flavored soft drinks, sugary confectionery products, rich bakery and flour confectionery products, pasta, ice cream, fruit ice, desserts, including flavored dairy products, complete dietary food mixtures, soups in amounts up to 50 mg/kg; in glazed fruits and vegetables, canned (colored) fruits, dry snacks based on potatoes, grains or starch, with extruded or exploded spices, alcoholic drinks, flavored wines and drinks based on them, fruit wines (still and sparkling), cider in amount up to 200 mg/kg; in decorative coatings, sauces, seasonings (dry and paste), pickles, etc., salmon fish, surimi minced fish in amounts up to 500 mg/kg; in flavored processed cheeses, pastes - fish and crustaceans, smoked fish, dry snacks based on potatoes, grains or starch, with spices except for extruded or exploded spicy snacks, solid biologically active food additives, meat and fish analogues based on vegetable proteins, in canned pea puree, in bitter soda drinks, bitter wine, made according to recipes approved by the State Sanitary and Epidemiological Supervision of the Ministry of Health of the Russian Federation, in quantities up to 100 mg/kg; in mustard, fish caviar, liquid biologically active food additives in amounts up to 300 mg/kg; in semi-finished crustacean products, boiled in quantities up to 250 mg/kg; edible coatings for cheeses and sausages in quantities according to TI individually or in combination with other permitted dyes (clauses 3.10.8, 3.10.16, 3.11.1 SanPiN 2.3.2.1293-03); for retail sale, including for Easter eggs (clause 2.25 of SanPiN 2.3.2.1293-03). Tartrazine refers to the sodium salt. Calcium and potassium salts and aluminum varnish are also allowed. |
| Application | Water-soluble dye alone or in mixtures with other dyes is used for coloring confectionery, ice cream, drinks, etc. in an amount of 0.05-0.5 g/kg. Mixing with blue dyes produces a green tint, which when added with red dyes produces colors ranging from brown to black. The shade of aluminum varnish can be changed by mixing it with varnishes of the above-mentioned dyes. Varnish is used primarily for coloring dragees. Other applications: approved in the EU and USA and used for coloring all medicines; |
CALCIUM TARTRATE E354
- Calcium tartaric acid, or calcium tartrate, is an E354 food additive that belongs to the class of antioxidants.
Antioxidants are added to foods to preserve them longer and protect them from bitterness and oxidation. They can be either of natural origin or chemically synthesized.
Tartrates are salts and esters of tartaric acid, actively used in the food industry.
Additive E 354 is used in the pharmaceutical and food industries. Its main property is the ability to regulate the level of acidity and at the same time it is safe for humans. So use it allowed in all countries, including baby food. In Russia, the list of products that include calcium tartrate includes flour, bakery products and alcoholic and non-alcoholic drinks.
Effect on the human body:Manufacturers do not deny that exceeding the permissible concentration of food additives can be harmful to health. It must be remembered that any substance affects the human body depending on its individual characteristics, as well as on the amount of the substance. Each supplement has its own acceptable daily intake (ADI). For E354 this dose is 30 mg per kilogram of body weight per day. In this quantity, the additive does not pose a danger to human health.
| Technological functions | Acidity regulator, acidifier, antioxidant synergist, salt substitute, emulsifying salt, color stabilizer, hardener. |
| Synonyms | Calcium tartrate; English calcium tartrate, calcium L(+)-tartrate; German Calcium-L(+)-tartrat; fr. L(+)-tartrate de calcium. |
| CAS# | 3164-34-9. |
| Chemical name | Calcium salt of 2,3-dihydroxybutanedioic acid. |
| Empirical formula | C 4 H 4 0 6 Ca 2H 2 0 (calcium tartrate dihydrate); C 4 H 4 0 6 Ca 4H 2 0 (calcium tartrate tetrahydrate) |
| Molecular mass | 224.18 (calcium tartrate dihydrate); 260.22 (calcium tartrate tetrahydrate). |
| Structural formula |  |
| Appearance | Colorless transparent crystals, white in mass. |
| Physicochemical characteristics | The water solution is dextrorotatory. Sol. in water; unsolvable in ethanol, oils, fats. |
| Natural spring | |
| Receipt | The interaction of L-tartaric acid with calcium hydroxide or calcium carbonates. Impurities: malates, other tartrates, oxalates. |
| Metabolism and toxicity | |
| Hygienic standards | DSP 30 mg/kg body weight per day in terms of C(+)-tartaric acid. Codex: approved as an acidity regulator for jams, preserves, jellies in amounts up to 3 g/kg individually or in combination with TARARTIC ACID, FUMARIC ACID and its salts. Permitted in the Russian Federation. |
| Application | Tartrates form stable complexes with iron and heavy metals and thus act as antioxidant synergists. They are sometimes used as melting salts in the production of processed cheese. The slow release of potassium and calcium from their salts regulates the rate of gelation of carrageenan, alginate and pectin gels. Calcium tartrate can be used to thicken plant tissue. Other applications: |
SODIUM-POTASSIUM TARTRATE E 337
Food additive E 337 - Potassium sodium tartrate (Segnet salt) used in the food industry as a stabilizer and complexing agent. It also enhances the effect of antioxidants and regulates acidity.
Rochelle salt E337 was named in honor of its creator, French pharmacist Pierre Seignet. He discovered it in the mid-17th century. Antioxidant, has a salty, cooling taste. It is included in the registers of food additives in almost all countries of the world.
The food additive E337 is widely used in the food industry as an acidity regulator in soups and broths. In citrus marmalades, jams and jellies, antioxidants are added in amounts of up to 3 grams per kilogram of product. In the Russian Federation, potassium sodium tartrate is used in canning vegetables and fruits, baking bakery and confectionery products. When making processed cheese, the food additive E337 is used as a melting salt. The antioxidant is able to regulate the rate of gelation of gels on pectin and alginates.
Effect on the human body:
When consuming products with the additive E 337 internally (baked goods and flour products, canned fruits and vegetables, drinks), you don’t have to worry - they are not harmful to health and are easily excreted in urine. But nevertheless, the permissible daily dosage is 30 mg/kg- should not be exceeded. In addition, such food is not recommended for people with disorders of the cardiovascular system (high blood pressure, heart failure), liver and kidneys.
This the additive is allowed not only in Russia, but also in many European countries.
| Technological functions | Acidity regulator, acidifier, antioxidant synergist, salt substitute, emulsifying salt, color stabilizer. |
| Synonyms | Potassium-sodium tartrate, sodium-potassium tartrate, potassium-sodium tartrate, sodium-potassium tartrate, potassium-sodium tartrate, Rochelle salt; English potassium sodium L(+)-tartrate, sodium-potassium L(+)-tartrate, potassium sodium dextro-tartrate, seignette salt, rochelle salt; German Natrium-Kaliumtartrat, Natrium-Kalium-L(+)-tar-trat; fr. tartrate de sodium-potassium, L(+)-tartrate de sodium-potassium. |
| CAS# | 6381-59-5. |
| Chemical name | Sodium-potassium salt of 2,3-dihydroxybutanedioic acid. |
| Empirical formula | C 4 H 4 0 6 NaK 4H 2 0 |
| Molecular mass | 282,23 |
| Structural formula |  |
| Organoleptic properties | Colorless transparent crystals, white in mass, have a salty, tongue-cooling taste. |
| Physicochemical characteristics | The water solution is dextrorotatory. Sol. in water (1 g in 1 ml); unsolvable in ethanol, oils, fats. |
| Natural spring | In many types of vegetables and fruits in the form of tartaric acid. |
| Receipt | The interaction of L-tartaric acid with sodium hydroxide and potassium hydroxide or sodium and potassium carbonates. Impurities: malates, other tartrates, oxalates. |
| Specifications |  |
| Metabolism and toxicity | According to tartaric acid content. |
| Hygienic standards |
DSP 30 mg/kg There are no dangers according to GN-98. Codex: approved as an acidity regulator for the following food products: soups, broths in amounts up to 250 mg/kg of finished product; jams, preserves, jellies, citrus marmalades up to 3 g/kg individually or in combination with and its salts to maintain pH between 2.8 and 3.5; GMP margarines. In the Russian Federation, canned fruits and vegetables are allowed in quantities according to technical specifications(clause 3.1.18 SanPiN 2.3.2.1293-03); in wines, drinks, food concentrates and other products, in bakery and flour confectionery products in quantities according to TI individually or in combination with tartaric acid and tartrates (clause 3.2.3,3.6.52 SanPiN 2.3.2.1293-03). |
| Application |
Tartrates form stable complexes with iron and heavy metals and thus act as antioxidant synergists. They are sometimes used as melting salts in the production of processed cheese. The slow release of potassium and calcium from their salts regulates the rate of gelation of carrageenan, alginate and pectin gels. Sodium potassium tartrate can be used as a salt substitute. Rochelle salt E 337 is used as an antioxidant in food preservation and the bakery industry. Rochelle salt is added to baking mixtures as a leavening agent. The field of application of the E337 additive is not limited to the food industry. Due to its electrophysical properties, sodium potassium tartrate has been used in the production of equipment. In particular, E337 was found in the pickups of telephone handsets, microphones, gramophones, and hearing aids. In the second half of the 20th century, Rochelle salt was increasingly used in the manufacturing process of electrical equipment. Additive E 337 is also used in the process of silvering mirrors, as a demulsifier for aqueous solutions in organic synthesis. In chemical laboratories, potassium sodium tartrate is necessary for the detection of sugars and proteins. Rochelle salt E337 is also used in medicine, as part of various drugs, including effervescent, instant drugs, and also as a laxative. Approved for use in all countries of the world. Other applications: in the production of pharmaceuticals. |
POTASSIUM TARTRATES E336
(i) 1-SUBSTITUTED POTASSIUM TARTRATE; (ii) 2-Substituted Potassium Tartrate
- Potassium tartrate (Potassium tartrate, dipotassium tartrate) is a medium salt of tartaric acid with the chemical formula C4H4K2O6;
- Potassium bitartrate (Potassium bitartrate) is an acidic salt of tartaric acid. Chemical formula KC4H5O6. Also known as cream of tartar, cream of tartar. Found in the juice of many berries.
Potassium tartrates - food additive E336, used in food products as an antioxidant, acidifier, acidity regulator, emulsifier. Additive E336 also stabilizes the color of products and enhances the effect of antioxidants.
Additive E336 is a mixture consisting of two organic substances that are similar in their physical and chemical properties:
They are very often confused with each other, so it is worth noting that dipotassium tartrate and potassium bitartrate are different compounds.
The use of this food additive is permitted in the Russian Federation and Ukraine.
Potassium tartrates are successfully used in winemaking and in the production of food additive E334 - tartaric acid. You can find potassium salts of tartaric acid in instant soups, jellies and candies with jelly filling, jams and marmalades. Cream of tartar is added as a leavening agent to confectionery and baked goods.
Since the food antioxidant E336 is of natural origin, the harm from its effect on the human body is negligible. The nutritional supplement has an effect on the cardiovascular system, reducing the flow of venous blood. This property of cream of tartar can relieve the condition of patients with varicose veins of the legs and colon. Potassium tartrates have a mild diuretic and laxative effect, salts help regulate the functioning of the gallbladder. It is not recommended to eat cream of tartar for diarrhea and flatulence.
| Technological functions | |
| Synonyms | Monopotassium tartrate and dipotassium tartrate, potassium tartrate and acid tartrate, monopotassium tartrate - potassium hydrogen tartrate, cream of tartar; English potassium L(+) tartrate, monopotassium tartrate, dipotassium tartrate; German Kaliumtartrat, Kalium-L(+)-tartrat; fr. tartrate de potassium, L(+)-tartrate de potassium. |
| CAS# | 868-14-4 (monopotassium tartrate); 6100-19-2 (dipotassium tartrate). |
| Chemical name | Potassium salts of 2,3-dihydroxybutanedioic acid |
| Empirical formula | C 4 H 5 0 6 K (monopotassium tartrate); C 4 H 4 0 6 K2 1/2 H2O (dicotassium tartrate). |
| Molecular mass | 188.18 (monopotassium tartrate); 235.28 (dicotassium tartrate). |
| Structural formula |  |
| Appearance | White crystals. |
| Physicochemical characteristics | Dextrorotatory. Sol. in water; unsolvable in ethanol, oils, fats. |
| Natural spring | In many types of vegetables and fruits in the form of tartaric acid. |
| Receipt | Interaction of L-tartaric acid with caustic potassium or potassium carbonates. Impurities: malates, other tartrates, oxalates. |
| Metabolism and toxicity | According to tartaric acid content. |
| Hygienic standards | ADI 30 mg/kg body weight per day in terms of L(+)-tartaric acid. Codex: allowed as acidity regulators for food products: soups, broths in amounts up to 250 mg/kg of finished product; jams, preserves, jellies, citrus marmalades up to 3 g/kg individually or in combination with tartaric acid, fumaric acid and its salts to maintain pH between 2.8 and 3.5; GMP margarines; grape juice and concentrated grape juice, preserved by physical methods only, GMP. In the Russian Federation, canned fruits and vegetables are allowed in grape juice in quantities according to TI (clauses 3.1.4, 3.1.18 SanPiN 2.3.2.1293-03); in wines, drinks, food concentrates and other products, in bakery and flour confectionery products in quantities according to TI individually or in combination with tartaric acid and tartrates (clause 3.2.3,3.6.52 SanPiN 2.3.2.1293-03). |
| Application | Tartrates form stable complexes with iron and heavy metals and thus act as antioxidant synergists and color stabilizers. They are sometimes used as melting salts in the production of processed cheese. Cream of tartar is convenient as a slow-acting acidifier in baking powders and as part of baking powder. The slow release of potassium from tartrate regulates the rate of gelation of carrageenan, alginate and pectin gels. Potassium tartrate allows you to quickly precipitate the cream of tartar from young wine. Other applications: in the production of pharmaceuticals. |
SODIUM TARTRES E335
(i) SODIUM TARTRATE, 1-SUBSTITUTED; (ii) SODIUM TARTRATE, 2-Substituted
Food additive E335– belongs to the group of natural antioxidants, also plays the role of an acidity regulator, salt substitute, acidifier, emulsifier, fixative and stabilizer of coloring agents. Protects products from rancidity, increases their shelf life, and promotes color fastness.
Sodium tartrate is added to instant soups and dry broths as an acidity regulator. Sodium tartaric acid is widely used in the production of confectionery, in candies with jelly filling, jellies and jams, preserves, fillers, marmalade, canned vegetables and fruits. In some quantities, the food additive E335 is used in the production of light oils and margarines.
Sodium tartrate is approved for use in the food industry in most countries, including Ukraine and the Russian Federation.
Effect on the human body:
The food additive E335 does not pose a danger to the human body when consumed in reasonable quantities.
The maximum permissible daily dose of its consumption is 30 milligrams per kilogram of human weight. You can be poisoned by salt vapors; to prevent poisoning from occurring, the concentration of sodium tartrate in the air should not exceed 10 milligrams per cubic meter. If this concentration is exceeded, respiratory tract burns may occur.
| Technological functions | Acidity regulators, acidifiers, antioxidant synergists, salt substitutes, emulsifying salts, color stabilizers. |
| Synonyms | Monosodium tartrate and disodium tartrate, sodium tartrate and sodium tartrate, sodium hydrogen tartrate (1-substituted sodium tartrate); English sodium L(+)-tartrate, monosodium tartrate, diso-dium tartrate; German Natriumtartrat, Natrium-L(+)-tartrat; fr. tartrate de sodium, L(+)-tartrate de sodium. |
| CAS# | 526-94-3 (monosodium tartrate); 868-18-8 (disodium tartrate); 6106-24-7 (disodium tartrate dihydrate). |
| Chemical name | Sodium salts of 2,3-dihydroxybutanedioic acid. |
| Empirical formula | C 4 H 5 0 6 Na- H 2 0 (monosodium tartrate); C 4 H 4 0 6 Na 2 (disodium tartrate); C 4 H 4 0 6 Ka 2 2 H 2 0 (disodium tartrate dihydrate). |
| Molecular mass | 190.10 (monosodium tartrate); 194.06 (disodium tartrate); 230.08 (disodium tartrate dihydrate). |
| Structural formula |  |
| Appearance | White crystals. |
| Physicochemical characteristics | Dextrorotatory. Sol. in water (1 g in 3 ml); unsolvable in ethanol, oils, fats. |
| Natural spring | In many types of vegetables and fruits in the form of tartaric acid. |
| Receipt | Interaction of L-tartaric acid with sodium hydroxide or sodium carbonates. Impurities: malates, other tartrates, oxalates. |
| Specifications | Sodium tartrate 2-substituted:  |
| Metabolism and toxicity | According to tartaric acid content. |
| Hygienic standards | DSP 30 mg/kg body weight per day in terms of C+)-tartaric acid. Hazards according to GN-98: MPC in the air of the working area 10 mg/m3, hazard class 3. Codex: approved as acidity regulators for the following foods: soups, broths in amounts up to 250 mg/kg of finished product; jams, preserves, jellies, citrus marmalades up to 3 g/kg individually or in combination with and its salts to maintain pH between 2.8 and 3.5; GMP margarines. In the Russian Federation are allowed in jams, jellies, marmalades and other similar products, including low-calorie, canned fruits and vegetables in quantities according to TI (clauses 3.1.6, 3.1.18 SanPiN 2.3.2.1293-03); in wines, drinks, food concentrates and other products, in bakery and flour confectionery products in quantities according to TI individually or in combination with tartaric acid and tartrates (clause 3.2.3, 3.6.52 SanPiN 2.3.2.1293-03). |
| Application | Cm. . |
CONTAINERS GUM E417
Tary gum E417 is a stabilizing substance designed to maintain the viscosity and consistency of food products. For example, pectin has a similar effect. Additive E417 belongs to the group of foam stabilizers, which are good emulsifiers and are added to liquid products to form and retain foam.
The main and most important quality of E 417 is increased strength and greater elongation. The gum mixes well with various substances and forms stable suspensions and elastic gels. Substances based on tara gum are thermoreversible.
The properties of container gum can be compared with the properties of guar gum; it is highly soluble in water, but a heated solution of container gum has a higher viscosity value and makes it possible to stabilize fine particles for a longer period.
In Russia, the additive E417 is approved for use in some products according to their manufacturing technology. At the same time, the maximum permissible amount for oral consumption has not been established, although previously it was 0.25 mg/kg body weight per day.
Effect on the human body:
Currently this supplement is recognized as completely safe for humans. But with increased concentrations, some discomfort from the gastrointestinal tract (flatulence and bloating) is possible.
| Technological functions | Thickener, stabilizer |
| Synonyms | Peruvian tree seed gum, container; English tara gum, tara, Peruvian gum; German Taga, Tarakernmehl, Taragummi, Peruanischesjohan-nisbrotkernmehl; fr. gomme de tara, tara. |
| CAS# | 39300-88-1 (container, modified container); 11078-30-1 (D-galacto-D-mannan). |
| Molecular mass | up to 300,000. |
| Compound | Neutral galactomannan, consisting of D-mannose and D-galactose in a ratio of 3:1. |
| Structural formula | The linear backbone consists of mannose residues linked by β-(1,4) glycosidic bonds; every third mannose is linked to galactose via an α-(1,6) bond. |
| Appearance | Powder from white to yellowish color. |
| Physicochemical characteristics | The quality is described by the content of galactomannans and the viscosity of the 1% solution: 2.5-3.5 Pa s (prepared in the cold), 3.0-5.0 Pa s (prepared by heating). Chorus. sol. in water; unsolvable in ethanol, org. solvents. |
| Natural spring | The endosperm of the shrub seeds contains Caesalpina spinosa of the Leguminosae family. |
| Receipt | Separating the seed peel (38-40%) and embryos (38-40%), grinding the isolated endosperm (about 20%). Impurities: remnants of seed skins and germs. |
| Specifications |
 |
| Metabolism and toxicity | It is not broken down by digestive enzymes; intestinal microflora can only partially cleave and destroy galactose side chains. |
| Hygienic standards | Chipboard not defined. Hygienic standards for quality and safety (SanPiN 2.3.2.1078-01): |
| Application | Tara gum can be used instead of guar gum or locust bean gum. The synergistic gel strengthening of agar, carrageenan and xanthan is weaker than that of locust bean gum. Tara gum is mainly used in gelling mixtures with xanthan, gellan, carrageenan, etc. |
| Product forms | Typically, commercial gum contains 80-85% galactomannans. |
TAUMATIN E 957
Thaumatin is present in the fruits of the Thaumatococcus danielli shrub, common in West Africa. When finished, it is a cream-colored, odorless powder. It has a rich sweet taste (it is about 2000 times sweeter than sucrose). But its taste is noticeably different from sugar, and the taste of Thaumatin appears gradually and lasts a long time.
In the world food anti-flaming E957 Thaumatin is approved in many countries, among them the Russian Federation, the European Union, Canada, Japan, the USA, Australia, Israel, etc. At the same time, it functions as a sweetener and, in small doses, a taste and aroma enhancer.
Effect on the human body:The daily dose of thaumatin for humans has not been described. It is believed that the food additive E957 is absolutely safe for health. Most countries allow the use of this sweetener on an industrial scale. In Russia, the substance did not pass the necessary tests, which is why it does not have permission for use in the food industry.
| Technological functions | Sweetener, flavor and aroma enhancer. |
| Synonyms | English thaumatin, katemfe; German Thaumatin; fr. thaumatine. |
| CAS# | 53850-34-3. |
| Compound | A polypeptide of 207 amino acid residues. |
| Organoleptic properties | A creamy, odorless powder with a strong sweet taste (several hundred times sweeter than sucrose), which does not appear immediately, but persists for a very long time. There is a licorice taste. |
| Physicochemical characteristics | Chorus. sol. in water; unsolvable in fatty solvents. |
| Natural spring | Ripe fruits of the African catemphe bush Thaumatococcus danielli (Maranthaceae). |
| Receipt | Extracting katemphe fruits with water. Impurities: other substances extracted from fruits. |
| Specifications |  |
| Metabolism and toxicity | It breaks down like a protein, no side effects were found. |
| Hygienic standards | Chipboard not defined. There are no dangers according to GN-98. Legislative approvals for use in food products are available in Australia, Japan, Canada, and the USA. EU: approved for sweetening the following products: confectionery products based on cocoa or dried fruits, sugar products, ice cream, all low-calorie or sugar-free products in quantities up to 50 mg/kg; sugar-containing chewing gum up to 10 mg/kg; biologically active and other food additives up to 400 mg/kg; table sweeteners QS. In the Russian Federation it is allowed as an additive that enhances and modifies the taste and aroma of a food product, in chewing gum with sugar in amounts up to 10 mg/kg; in desserts in amounts up to 5 mg/kg; in soft drinks with flavors in amounts up to 0.5 mg/l (clause 3.14.10 of SanPiN 2.3.2.1293-03); in the case of combination with acesulfame potassium, aspartame and thaumatin in the manufacture of chewing gum, the maximum level of each should be proportionally reduced, i.e. the total mass should be no more than 100%; as a sweetener in confectionery products with reduced calorie content or without added sugar, including those based on starch, cocoa, dried fruits; in chewing gum without added sugar; in ice cream (except milk and cream), fruit ice with reduced calorie content or without added sugar in amounts up to 50 mg/kg; in biologically active food supplements: vitamins and minerals in the form of syrups and chewable tablets in amounts up to 400 mg/kg (clause 3.15.8 of SanPiN 2.3.2.1293-03); for retail sale (clause 2.22 of SanPiN 2.3.2.1293-03). |
| Application | Typically, thaumatin is used as a sweetener for special types of chewing gum. Sweetness may be lost when the protein denatures. At very low dosages, thaumatin exhibits flavor and aroma enhancer properties, so that the threshold concentrations of some aromas are clearly reduced. |
| Product forms | Powder or concentrated solutions. |
THERMALLY OXIDIZED SOY OIL WITH MOHO- AND DIGLYCERIDES OF FATTY ACIDS E 479
- The fat remaining after frying in a frying pan, which all housewives are so afraid of, is nothing more than additive E 479.
Thermally oxidized oils are used to prevent foaming and crystallization of fats and oils. E479b is used as an emulsifier for the production of emulsions, margarines, and edible waxes.
E479b is included in:
- ready-made fats for deep frying,
- margarines,
- oils and fats with long shelf life.
The permissible daily intake of additive E 479b is no more than 15 mg. The additive does not have permission for use in food production in the Russian Federation. Thermally oxidized oils are not allergens. Additive E479b is not used in the production of baby food. People with diseases of the stomach and intestinal tract should be careful when consuming products with the addition of thermally oxidized oils.
| Technological functions | Emulsifier, separator, antifoaming agent. |
| Synonyms | Oxystearin, thermo-oxidized soybean oil; English oxystearin, thermooxydated sojaoils, TOSOM; German geblasene Ole, Oxistearine, Thermoxydierte Ole; fr. oxystearine |
| Compound | Reaction mixtures of cross-linked fatty acid esters with polymerized glycerol in an excess of neutral fat, additionally esterified with monoglycerides or free glycerol. |
| Organoleptic properties | A yellow to light brown fatty or waxy substance. |
| Physicochemical characteristics | T pl above 40°C. Chorus. sol. in hot oil; Wed sol. in hot water; unsolvable in cold water, fat, oil. |
| Natural spring | Contained in used deep fat as an undesirable product, its quantity characterizes the degree of deterioration of the deep fat. |
| Receipt | When pure fat is gently oxidized by air, “oxystearin” is formed. With rapid oxidation at 200°C, polymerization products are formed - “thermally oxidized soybean oil.” If monoglycerides or glycerol are present during the oxidation process, or thermally oxidized soybean oil is esterified with monoglycerides or free glycerol, then “thermal oxidized soybean oil with mono- and diglycerides of fatty acids” is obtained. Impurities: Reaction mixtures may contain hydroxy acids, keto acids and various peroxides or sulfites and sulfates added to destroy the peroxides. |
| Specifications |  |
| Metabolism and toxicity | The digestibility of these products is low. In normal household use of fats for frying, deep-frying, etc. the same products are formed. At this level, their intended use as a food additive should not pose a health hazard, even if the available data are (yet) insufficient for a full toxicological assessment. |
| Hygienic standards | ADI 25 mg/kg body weight per day (for oxystearin). There are no dangers according to GN-98. Codex: oxystearin is allowed in 12 standards for edible vegetable oils as a crystallization inhibitor in amounts up to 1250 mg/kg. EU: in working documents E479 is divided into: thermo-oxidized soybean oil (E479a) - allowed for QS release emulsions; thermo-oxidized soybean oil, esterified with mono- and diglycerides (E479b) - in QS baking margarines. (Clause 3.6.8 SanPiN 2.3.2.1293-03). |
| Application | Oxystearins are added to edible fats and oils as an antifoaming agent and to prevent crystallization (50-100 mg/kg). Thermally oxidized soybean oil is used in release waxes and emulsions, and as an antifoam agent. Polymerized fats containing mono- and diglycerides are used as emulsifiers in release emulsions and margarines for baking. Other applications: as a foam retarder or antifoam agent. |
| Product forms | Three different products are sold under the names “hydroxystearin”, “thermal oxidized soybean oil”, “thermal oxidized soybean oil with mono- and diglycerides of fatty acids”, and the names are sometimes confused. In addition, there are mixed products. |
TIABENDAZOLE E233
Many countries, including the Russian Federation, allow the use of thiabendazole in the food industry. But the states that are part of the European Union recognize it only as a pesticide.
| Technological functions | Preservative. |
| Synonyms | Mintezol; English thiabendazole; German Thiabendazol, Tiabendazol (in pharmaceuticals); fr. thiabendazole. |
| CAS# | 148-79-8. |
| Chemical name | 2-(4-Thiazolyl)-benzimidazole. |
| Empirical formula | C10H7N3S |
| Molecular mass | 210,25 |
| Structural formula |  |
| Organoleptic properties | White crystalline powder, odorless and tasteless. |
| Physicochemical characteristics | Boil temperature 304-305°C. Sol. in water (the higher the solubility, the lower the pH, maximum at pH 2.2); unsolvable in alcohols. |
| Receipt | Condensation of 4-cyanothiazole with ortho-phenylenediamine in the presence of an acid catalyst, followed by precipitation with alcohol. Impurities: carriers and solvents. |
| Specifications |  |
| Metabolism and toxicity | The rate of absorption of thiabendazole is insignificant, so it can be used in medicine. A small amount of absorbed substances is excreted from the body in the urine in the form of glucoronides. |
| Hygienic standards | There is no chipboard. It does not have permission in the Russian Federation. |
| Application | Thiabendazole is a fungistatic, i.e. a mold preventive agent, although its mechanism of action against molds is unknown. It is added to the emulsion or solution in an amount of 0.1-0.45% to treat plants, primarily citrus fruits and bananas, before and after harvest. Part of the residual amount of thiabendazole (5-12%) from the peel of citrus fruits passes into the pulp, and part (7-14%) ends up on the hands. Residues of thiabendazole are good. can be removed with both warm and cold water. In the EU, thiabendazole is currently recommended for use only as a pesticide. Other applications: In medical practice, thiabendazole is used as a fungistatic agent in disinfectant sprays for the skin and as an anthelmintic. |
| Product forms | Individual substance, wax with 0.1 -0.5% thiabendazole, concentrates for immersion baths with or without wax, varnish-like pastes. |
THIOUREA
| Technological functions | Preservative. |
| Synonyms | Thiocarbamide. |
| CAS# | 62-56-6. |
| Empirical formula | CH4N2S |
| Molecular mass | 76,14 |
| Structural formula |  |
| Properties and application | Aqueous solutions of thiocarbamide at a concentration of 500 mg/kg can reduce or slow down enzymatic browning and microbial spoilage of (cut) fruits, but are not used in food products due to toxicity (thiourea is a carcinogen and also blocks the thyroid gland). The maximum permissible concentration of thiourea in the air of the working area is 0.3 mg/m3, hazard class 2; |
SODIUM THIOSULPHATE E 539
- Previously food emulsifier E 539 sodium thiosulfate used in the food industry as an antioxidant and complexing agent, for example, added to iodized salt and flour. But in 2010 it was banned in Russia. This additive is also not approved in the EU.
In the food industry, sodium thiosulfate is used mainly as an antioxidant for iodized salt and a quality improver for flour and bread. As a flour improver, food additive E 539 can act as an independent component or be used in combination with a number of other similar improvers. Sodium thiosulfate is included in iodized salt in a proportion of no more than 250 mg per 1 kg of salt. In the baking process, its mass fraction does not exceed 0.002 percent of the total mass of flour or up to 50 mg per 1 kg of product. In addition to the food industry, the E 539 additive is widely used in medicine.
It is part of complex baking improvers, and is also used as an individual baking improver with restorative action. Restorative improvers are recommended to be used to change the rheological properties of dough made from wheat flour with excessively strong or short-tearing gluten. At the same time, the volumetric yield of bread increases, the crumb becomes more elastic and loose, and cracks and tears on the surface of the product are smoothed out.
When used individually, sodium thiosulfate is added together with baker's yeast in an amount of 0.001-0.002% by weight of flour, depending on the method of baking bread (hearth or tin). To ensure an accurate dosage of the improver, prepare its aqueous solution in a ratio of 1:20. The solution can be stored for no more than a day in a closed container made of non-corrosive material.
Sodium thiosulfate is also used to stabilize iodine in the production of iodized salt in amounts up to 250 mg/kg.
Other applications: in pharmaceuticals as an anti-inflammatory agent, an antidote for poisoning with arsenic, mercury, etc.; in textileName: Potassium sodium tartrate E337
Other names: E337, E-337, English: E337, E-337, Sodium potassium tartrate
Group: Food additive
Type: Antioxidants, antioxidants
Effect on the body: safe
Approved in countries: Russia, Ukraine, EU
Rochelle salt E337 (Sodium potassium tartrate, potassium sodium tartrate) was named in honor of its creator, French pharmacist Pierre Seignet. He discovered it in the mid-17th century. Antioxidant, has a salty, cooling taste. It is included in the registers of food additives in almost all countries of the world.
In appearance, these are crystals with a range of colors from blue to colorless. The decomposition process of the additive occurs already at a temperature of 55.6 ° C, and water of crystallization evaporates from the substance. Easily dissolves in water, has high hygroscopicity, and during dissolution in water it partially precipitates.
Rochelle salt E337 has specific electrical properties; it can carry out polarization in temperature gradations. Some derivatives of potassium sodium tartrate (its tetrahydrate) have piezoelectric properties.
Application:
Rochelle salt E337 is used as an antioxidant in food preservation and the bakery industry. Rochelle salt is added to baking mixtures as a leavening agent. The field of application of the E337 additive is not limited to the food industry. Due to its electrophysical properties, sodium potassium tartrate has been used in the production of equipment. In particular, E337 was found in the pickups of telephone handsets, microphones, gramophones, and hearing aids. In the second half of the 20th century, Rochelle salt was increasingly used in the manufacturing process of electrical equipment.
Additive E-337 is also used in the process of silvering mirrors, as a demulsifier for aqueous solutions in organic synthesis. In chemical laboratories, potassium sodium tartrate is necessary for the detection of sugars and proteins.
Rochelle salt E337 is also used in medicine, as part of various drugs, including effervescent, instant drugs, and also as a laxative. Approved for use in all countries of the world.
Effect on the human body:
There is currently no information about the negative effects on the human body. There is information that Rochelle salt E337 was widely used in medicine to treat disorders of the digestive system, as a laxative. Normalizes digestive processes in the body, participates in the process of removing toxins. For a long time, sodium potassium tartrate E337 has been used as a morning tonic in Seydlitz homeopathic powder. The daily dose for humans has not been described.
Potassium-sodium tartrate GOST 5845-79
KNaC 4 H 4 O 6 4H 2 O
Rochelle salt- tetrahydrate of double sodium-potassium salt of tartaric acid ( sodium potassium tartrate). Named after the French pharmacist Pierre Seignet (fr. Pierre Seignette), 1660-1719 (other sources indicate the name of the pharmacist Elie Seigner (1632-1698), as well as the years of obtaining salt - 1672 and 1675).
Chemical properties and application
Since sodium potassium tartrate is a salt of tartaric acid, several optical isomers correspond to it. Only L-(+)-tartaric acid occurs in nature
The tetrahydrate is highly soluble in water (54 g/100 g) at 15 °C, at 30 °C 1390 g/l), and the salt is hygroscopic. However, the salt as such is obviously poorly soluble, since it precipitates during the preparation reaction.
Sodium potassium tartrate is a component of Fehling's liquid, in which it is used to detect sugars. Rochelle salt is also used in silvering mirrors using the Heinrichson method. In addition, this salt is used in organic synthesis as a demulsifier in aqueous solutions, usually in reactions involving aluminum hydride. Finally, the solution for the determination of proteins by the biuret method also contains sodium potassium tartrate.
In the laboratory, this salt is obtained by precipitation in fine crystalline form from a hot solution of potassium acid tartrate by adding a stoichiometric amount of Na 2 CO 3.
Potassium sodium tartrate- acidity regulator, acidifier, antioxidant synergist, salt substitute, emulsifying salt, color stabilizer.
Potassium sodium tartrate physical and chemical properties
Colorless transparent crystals, white in mass, have a salty, tongue-cooling taste. Soluble in water (1 g in 1 ml); insoluble in ethanol, oils, fats.
Potassium sodium tartrate natural source
In many types of vegetables and fruits in the form of tartaric acid.
Preparation of potassium sodium tartrate
The interaction of L-tartaric acid with sodium hydroxide and potassium hydroxide or sodium and potassium carbonates. Impurities: malates, other tarts, oxalates. According to tartaric acid content.
Daily intake of potassium sodium tartrate
ADI 30 mg/kg body weight per day in terms of L(+)-tartaric acid. There are no dangers according to GN-98.
Mention in standards and application in the food industry E-337
E-337 approved as an acidity regulator for the following food products: soups, broths in amounts up to 250 mg/kg of finished product; jams, preserves, jellies, citrus marmalades up to 3 g/kg individually or in combination with TARTARIC ACID, FUMARIC ACID and its salts to maintain pH between 2.8 and 3.5; GMP margarines. In the Russian Federation, it is allowed in canned fruits and vegetables in quantities according to TI (clause 3.1.18 of SanPiN 2.3.2.1293-03); in wines, drinks, food concentrates and other products, in bakery and flour confectionery products in quantities according to TI individually or in combination with tartaric acid and tartrates (clause 3.2.3,3.6.52 SanPiN 2.3.2.1293-03). Tartrates form stable complexes with iron and heavy metals and thus act as antioxidant synergists. They are sometimes used as melting salts in the production of processed cheese. The slow release of potassium and calcium from their salts regulates the rate of gelation of carrageenan, alginate and pectin gels. Sodium potassium tartrate can be used as a salt substitute.
Other uses of potassium sodium tartrate:
E-337 used in the production of pharmaceuticals, as well as for silvering mirrors, as a mouthwash, laxative