Elementary particles and their main characteristics. The structure of the atom and the characteristics of elementary particles
All bodies, including ourselves, are made up of tiny building blocks called atoms. There are as many types of such "bricks" as there are in nature chemical elements. A chemical element is a collection of atoms of the same type.
The idea that matter is built from the smallest "particles" was expressed by ancient Greek scientists. It was they who called these particles atoms (from the Greek word meaning "indivisible"). The ancient Greeks believed that atoms were shaped regular polyhedra: cube ("earth atoms"), tetrahedron ("fire atoms"), octahedron ("air atoms"), icosahedron ("water atoms"). More than twenty centuries passed before experimental confirmation of the idea of the atomistic structure of matter was obtained. This idea was finally established in science in the second half of the 19th century thanks to the successes of chemistry and molecular-kinetic theory. By the beginning of the 20th century, physicists already knew that atoms have dimensions of the order of 10-10 m and a mass of 10-27 kg. By this time, it became clear that atoms are not "indivisible" at all, that they have a certain internal structure, the solution of which will explain the periodicity of the properties of chemical elements, identified by Dmitry Ivanovich Mendeleev (1834-1907).
In 1903, shortly after the discovery of the electron, the English physicist Joseph John Thomson (1856-1940) proposed a model of the atom in the form of a positively charged sphere with a diameter of about 10-10 m, inside which electrons are interspersed (see Elementary particles). negative charge electrons compensated positive charge spheres. When the electrons vibrate about the center of the sphere, the atom emits light. Thomson believed that electrons were grouped into layers around a center.
In the model proposed by Thomson, the mass of an atom is evenly distributed over its volume. The erroneousness of this assumption was soon proved by the English physicist Ernest Rutherford (1871-1937). In 1908-1911, under his leadership, experiments were carried out on the scattering of a-particles (helium nuclei) by metal foil, and the particle freely passed through a thin foil, experiencing only minor deviations; however, in some rare cases (about one in 10,000) scattering of a-particles through an angle greater than 90° was observed.
"It was almost as incredible," Rutherford later recalled, "as if you fired a 15-inch projectile at a sheet of tissue paper, and the projectile would come back and hit you."
Experiments on the scattering of a-particles convincingly showed that almost the entire mass of the atom is concentrated in a very small volume - the atomic nucleus, whose diameter is approximately
10,000 times smaller than the diameter of an atom. Most a-particles fly past the massive nucleus without touching it, only occasionally colliding with it and "bouncing" back.
Rutherford's experiments served as the basis for the creation of the proton-neutron model of the atom. This model determines modern ideas about the structure of the atom.
So, in the center of the atom there is an atomic nucleus (its dimensions are about 10 "14 m); the rest of the volume of the atom is electrons. There are no electrons inside the nucleus (this became clear in the early 30s); the nucleus consists of positively charged protons and not having a charge of neutrons.The number of electrons in an atom is equal to the number of protons in the nucleus; this is the atomic number of a given chemical element (its serial number in the periodic system).The mass of an electron is about 2000 times less than the mass of a proton or neutron, so almost all the mass of an atom is concentrated in nucleus.Different electrons are bound to the nucleus to varying degrees;
some of them are "lost" relatively easily, with the atom turning into positive ion. By acquiring additional electrons, the atom becomes a negative ion.
In creating his model of the atom, Rutherford suggested that Coulomb forces act between negatively charged electrons and a positively charged nucleus. It is clear that the electrons cannot rest inside the atom, since they would then fall on the nucleus, therefore, according to Rutherford's assumption, the electrons move around the nucleus, just as the planets revolve around the sun. Therefore, Rutherford's model of the atom was called planetary.
A simple and visual planetary model of the atom has a direct experimental justification. It is absolutely necessary for explaining the experiment on the scattering of a-particles. But such a model contradicts the laws of mechanics and electrodynamics. It does not allow explaining the existence of the atom, its stability. After all, the movement of electrons in orbits occurs with acceleration, and very large. According to Maxwell's laws of electrodynamics, an accelerated charge must radiate electromagnetic waves with a frequency equal to the number of its revolutions around the nucleus per second. Radiation is accompanied by a loss of energy. Losing energy, the electrons should approach the core, just as a satellite approaches the Earth when braking in the upper atmosphere. As shown by completely rigorous calculations based on Newton's mechanics and Maxwell's electrodynamics, an electron must fall on the nucleus in a negligible time (about 10-8 s), and the atom must cease to exist.
In reality, nothing like this happens. Atoms are stable and in an unexcited state can exist indefinitely without emitting electromagnetic waves at all. This leads to the most important conclusion: the laws of classical physics are inapplicable to the phenomena of atomic scales.
The way out of an extremely difficult situation was found in 1913 by the great Danish physicist Niels Bohr (1885-1962), who introduced his famous quantum postulates that determine the structure of the atom and the conditions for its emission and absorption electromagnetic radiation. Here they are:
The first postulate: an atomic system can only be in special stationary, or quantum, states, each of which corresponds to a certain energy En. In a stationary state, an atom does not radiate.
This postulate is in clear contradiction with classical mechanics, according to which the energy of moving electrons can be any. It also contradicts Maxwell's electrodynamics, since it allows the possibility of accelerated motion without radiation of electromagnetic waves.
The second postulate: during the transition of an atom from one stationary state to another, a quantum of electromagnetic energy is emitted or absorbed.
The second postulate also contradicts Maxwell's electrodynamics, according to which the frequency of the emitted light is equal to the frequency of the electron's orbit. According to Bohr's theory, the frequency is associated only with the change in the energy of the atom.
ATOMIC NUCLEUS
According to the proton-neutron model, atomic nuclei consist of elementary particles of two types: protons and neutrons (see also Atom).
It is known that the proton charge is positive and equal to the charge electron.
The neutron has no electric charge, its mass is 1.00867 a.m.u. 1 atomic mass unit (a.m.u.) is equal to 1/12 of the mass of a carbon atom and is related to the kilogram by the ratio 1 a.m.u. =1.6605 10-27 kg; 1 amu corresponds to an energy of 931.5 MeV).
The number of protons in the nucleus is called the nuclear charge and is equal to the number of electrons in atomic shell, since the atom is generally neutral. Therefore, the number of protons in the nucleus is equal to the atomic number of the element ZB in the periodic table.
The mass number of the nucleus A is the sum of the number of protons Z and the number of neutrons N in the nucleus: A=Z+N.
Since the masses of the proton and neutron are close to each other, the mass number A is very close to the relative atomic mass of the element. Mass numbers can be determined by rough measurements of the masses of nuclei with instruments that do not have very high accuracy.
However, the relative atomic masses some elements are very different from an integer. So, for boron it is 10.81, for chlorine - 35.45. Why? It turns out that the nuclei of the same chemical element can differ in the number of neutrons with the same number of protons in the nucleus and electrons in the electron shell,
These nuclei have the same Chemical properties and are located in one cell of the periodic table. These are isotopes. Chemically simple natural substances are a mixture of isotopes. Thus, boron consists of a mixture of two isotopes: 20% of it is an isotope with mass number 10 (5 protons, 5 neutrons), and 80% - with a mass number of 11 (5 protons and 6 neutrons).
Elementary particles are particles that have this moment no internal structure found. Even in the last century, atoms were considered elementary particles. Their internal structure - nuclei and electrons - was discovered at the beginning of the 20th century. in the experiments of E. Rutherford. The size of atoms is about 10 -8 cm, nuclei are tens of thousands of times smaller, and the size of electrons is very small. It is less than 10 -16 cm, as follows from modern theories and experiments.
Thus, now the electron is an elementary particle. As for the nuclei, their internal structure was revealed shortly after their discovery. They are made up of nucleons - protons and neutrons. Nuclei are fairly dense: the average distance between nucleons is only a few times their own size. In order to find out what nucleons consist of, it took about half a century, however, at the same time, other mysteries of nature appeared and were solved.
Nucleons consist of three quarks, which are elementary with the same accuracy as an electron, i.e. their radius is less than 10 -16 cm. The radius of nucleons - the size of the area occupied by quarks - is about 10 -13 cm. Nucleons belong to a large family particles - baryons, composed of three different (or identical) quarks. Quarks can form triples in different ways, and this determines the differences in the properties of a baryon, for example, it can have a different spin.
In addition, quarks can combine into pairs - mesons, consisting of a quark and an antiquark. The spin of mesons takes integer values, while for baryons it takes half-integer values. Together baryons and mesons are called hadrons.
Quarks have not been found in free form, and according to currently accepted concepts, they can exist only in the form of hadrons. Before the discovery of quarks, hadrons were considered elementary particles for some time (and this name is still quite common in the literature).
The first experimental indication of the composite structure of hadrons were experiments on the scattering of electrons by protons at the Stanford (USA) linear accelerator, which could only be explained by assuming the presence of some point objects inside the proton.
It soon became clear that these were quarks, the existence of which was assumed even earlier by theorists.
Here is a table of modern elementary particles. In addition to six types of quarks (only five have so far appeared in experiments, but theorists suggest that there is also a sixth), this table lists leptons - particles to which the electron also belongs. The muon and (quite recently) the t-lepton have also been discovered in this family. Each of them has its own neutrino, so that the leptons naturally split into three pairs e, n e; m, n m ;t, n t .
Each of these pairs combines with the corresponding pair of quarks into a quadruple, which is called a generation. The properties of particles are repeated from generation to generation, as can be seen from the table. Only the masses differ. The second generation is heavier than the first, and the third generation is heavier than the second.
In nature, particles of the first generation are mainly found, and the rest are created artificially on charged particle accelerators or during the interaction of cosmic rays in the atmosphere.
In addition to spin 1/2 quarks and leptons, collectively called particles of matter, the table lists particles with spin 1. These are the quanta of the fields created by the particles of matter. Of these, the most well-known particle is the photon, a quantum of the electromagnetic field.
The so-called intermediate bosons W+ and W- , which have very large masses, were recently discovered in experiments on counter R-beams at energies of several hundred GeV. These are carriers of weak interactions between quarks and leptons. And finally, gluons are carriers of strong interactions between quarks. Like the quarks themselves, gluons have not been found in free form, but appear at intermediate stages of the reactions of creation and annihilation of hadrons. Recently, hadron jets generated by gluons have been detected. Since all the predictions of the theory of quarks and gluons - quantum chromodynamics - agree with experience, there is almost no doubt about the existence of gluons.
A particle with spin 2 is a graviton. Its existence follows from Einstein's theory of gravity, the principles quantum mechanics and the theory of relativity. It will be extremely difficult to detect the graviton experimentally, since it interacts very weakly with matter.
Finally, the table with a question mark shows particles with spin 0 (H-mesons) and 3/2 (gravitinos); they have not been found experimentally, but their existence is assumed in many modern theoretical models.
Elementary particles
| spin | 0? | 1/2 | 1 | 3/2 | 2? | ||||||
| title | Particles of matter | Field quanta | |||||||||
| quarks | leptons | photon | vector bosons | gluon | gravitino | graviton | |||||
| symbol | H | u | d | ne | e | g | Z | W | g | ||
| (weight) | (?) | (?) | (0,5) | (0) | (~95 GeV) | (~80 GeV) | (?) | (?) | |||
| symbol | With | s | nm | m | |||||||
| (weight) | (0?) | (106) | |||||||||
| symbol | t | b | n t | t | |||||||
| (weight) | (0?) | (1784) | |||||||||
|
baryonic |
0 | 1/3 | 1/3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Electric |
0,±1 | 2/3 | 1/3 | 0 | -1 | 0 | 0 | ±1 | 0 | 0 | 0 |
| color | - | 3 | 3 | - | - | - | - | - | 8 | - | - |
Hadrons - common name for particles participating in strong interactions . The name comes from the Greek word meaning "strong, large". All hadrons are divided into two large groups - mesons and baryons.
baryons(from the Greek word meaning "heavy") are hadrons with half-integer spin . The most famous baryons are proton and neutron . Baryons also include a number of particles with a quantum number, once called oddity. The unit of strangeness is possessed by the lambda baryon (L°) and the family of sigma baryons (S - , S+ and S°). Indices +, -, 0 indicate the sign of the electric charge or the neutrality of the particle. Baryons xy (X - and X°) have two units of strangeness. Baryon W - has a strangeness equal to three. The masses of the listed baryons are about one and a half times the mass of the proton, and their characteristic lifetime is about 10 -10 s. Recall that the proton is practically stable, while the neutron lives for more than 15 minutes. It would seem that heavier baryons are very short-lived, but on the scale of the microcosm, this is not so. Such a particle, even moving relatively slowly, with a speed equal to, say, 10% of the speed of light, manages to travel a distance of several millimeters and leave its mark in the detector of elementary particles. One of the properties of baryons that distinguish them from other types of particles can be considered the presence of a conserved baryon charge. This value was introduced to describe the experimental fact of constancy in all known processes of the difference between the number of baryons and antibaryons.
Proton- a stable particle from the class of hadrons, the nucleus of a hydrogen atom. It is difficult to say what event should be considered the discovery of the proton: after all, as a hydrogen ion, it has been known for a long time. The creation of the planetary model of the atom by E. Rutherford (1911), and the discovery of isotopes (F. Soddy, J. Thomson, F. Aston, 1906-1919), and the observation of hydrogen nuclei knocked out by alpha particles from nitrogen nuclei played a role in the discovery of the proton. (E. Rutherford, 1919). In 1925, P. Blackett received the first photographs of proton traces in a cloud chamber (see Nuclear radiation detectors), confirming the discovery of the artificial transformation of elements. In these experiments, the a-particle was captured by a nitrogen nucleus, which emitted a proton and turned into an oxygen isotope.
Together with neutrons, protons form the atomic nuclei of all chemical elements, and the number of protons in the nucleus determines the atomic number of a given element. The proton has a positive electric charge equal to the elementary charge, i.e., the absolute value of the electron charge. This has been verified experimentally with an accuracy of 10 -21 . proton mass m p \u003d (938.2796 ± 0.0027) MeV or ~ 1.6-10 -24 g, i.e. the proton is 1836 times heavier than the electron! From the modern point of view, the proton is not true elementary particle: it consists of two u-quarks with electric charges +2/3 (in units elementary charge) and one d-quark with electric charge -1/3. Quarks are interconnected by the exchange of other hypothetical particles - gluons, quanta of the field that carries strong interactions. The data of experiments in which the processes of scattering of electrons by protons were considered do indeed indicate the presence of point scattering centers inside protons. These experiments are in a certain sense very similar to Rutherford's, which led to the discovery of the atomic nucleus. As a composite particle, the proton has a finite size of ~ 10 -13 cm, although, of course, it cannot be represented as a solid ball. Rather, the proton resembles a cloud with a fuzzy boundary, consisting of emerging and annihilating virtual particles.
The proton, like all hadrons, participates in each of the fundamental interactions. So. strong interactions bind protons and neutrons in nuclei, electromagnetic interactions - protons and electrons in atoms. Examples of weak interactions are the beta decay of a neutron or the intranuclear transformation of a proton into a neutron with the emission of a positron and a neutrino (for a free proton, such a process is impossible due to the law of conservation and conversion of energy, since the neutron has a slightly larger mass). The proton spin is 1/2. Hadrons with half-integer spin are called baryons (from the Greek word for "heavy"). Baryons include a proton, a neutron, various hyperons (L, S, X, W) and a number of particles with new quantum numbers, most of which have yet to be discovered. To characterize baryons, a special number has been introduced - the baryon charge, equal to 1 for baryons, - 1 - for antibaryons, and O - for all other particles. The baryon charge is not a source of the baryon field; it was introduced only to describe the regularities observed in reactions with particles. These regularities are expressed in the form of the baryon charge conservation law: the difference between the number of baryons and antibaryons in the system is preserved in any reactions. The conservation of the baryon charge makes it impossible for the proton to decay, because it is the lightest of the baryons. This law is empirical in nature and, of course, must be tested experimentally. The accuracy of the law of conservation of baryon charge is characterized by the stability of the proton, the experimental estimate for the lifetime of which gives a value of at least 1032 years.
At the same time, in theories that combine all types of fundamental interactions, processes are predicted that lead to the violation of the baryon charge and to the decay of the proton. The lifetime of a proton in such theories is indicated not very accurately: approximately 1032 ± 2 years. This time is huge, it is many times longer than the time of the existence of the Universe (~ 2 10 10 years). Therefore, the proton is practically stable, which made possible the formation of chemical elements and, ultimately, the emergence of intelligent life. However, the search for proton decay is now one of the most important problems in experimental physics. With a proton lifetime of ~ 1032 years in a water volume of 100 m3 (1 m3 contains ~ 1030 protons), one proton per year should be expected to decay. It remains only to register this decay. The discovery of the decay of the proton will be an important step towards a correct understanding of the unity of the forces of nature.
Neutron- a neutral particle belonging to the class of hadrons. Opened in 1932 by the English physicist J. Chadwick. Along with protons, neutrons are part of atomic nuclei. Electric charge neutron q n is equal to zero. This is confirmed by direct measurements of the charge from the deflection of the neutron beam in strong electric fields, showing that |q n |<10 -20 e(here e- elementary electric charge, i.e., the absolute value of the electron charge). Indirect data estimate |q n |< 2*10 -22 е. Спин нейтрона равен 1/2. Как адрон с полуцелым спином, он относится к группе барионов. У каждого бариона есть античастица; антинейтрон был открыт в 1956 г. в опытах по рассеянию антипротонов на ядрах. Антинейтрон отличается от нейтрона знаком барионного заряда; у нейтрона, как и у протона, барионный заряд равен +1.
Like the proton and other hadrons, the neutron is not a true elementary particle: it consists of one u-quark with electric charge +2/3 and two d-quarks with a charge of - 1/3, interconnected by a gluon field.
Neutrons are stable only in stable atomic nuclei. A free neutron is an unstable particle that decays into a proton (p), an electron (e -) and an electron antineutrino. The neutron lifetime is (917 ± 14) s, i.e., about 15 min. Neutrons exist in free form in matter even less due to strong absorption by their nuclei. Therefore, they arise in nature or are obtained in the laboratory only as a result of nuclear reactions.
According to the energy balance of various nuclear reactions, the value of the mass difference between the neutron and proton is determined: m n- m p (1.29344 ±0.00007) MeV. By comparing it with the mass of the proton, we obtain the mass of the neutron: m n = 939.5731 ± 0.0027 MeV; it corresponds m n ~ 1.6-10 -24.
The neutron participates in all kinds of fundamental interactions. Strong interactions bind neutrons and protons in atomic nuclei. An example of a weak interaction is the beta decay of a neutron.
Does this neutral particle participate in electromagnetic interactions? The neutron has an internal structure, and in the case of general neutrality there are electric currents in it, leading, in particular, to the appearance of a magnetic moment in the neutron. In other words, in a magnetic field, a neutron behaves like a compass needle. This is just one example of its electromagnetic interaction. The search for the electric dipole moment of the neutron, for which an upper limit was obtained, acquired great interest. Here the scientists of the Leningrad Institute of Nuclear Physics of the Academy of Sciences of the USSR managed to carry out the most effective experiments; Searches for the dipole moment of neutrons are important for understanding the mechanisms of violation of invariance with respect to time reversal in microprocesses.
Gravitational interactions of neutrons were observed directly from their incidence in the Earth's gravitational field.
A conditional classification of neutrons according to their kinetic energy has now been adopted:
slow neutrons (<10 5 эВ, есть много их разновидностей), быстрые нейтроны (10 5 ¸10 8 эВ), высокоэнергичные (>10 8 eV). Very slow neutrons have very interesting properties.
(10 -7 eV), which are called ultracold. It turned out that ultracold neutrons can be accumulated in "magnetic traps" and even their spins can be oriented there in a certain direction. Using magnetic fields of a special configuration, ultracold neutrons are isolated from absorbing walls and can "live" in a trap until they decay. This allows many subtle experiments to study the properties of neutrons. Another method of storing ultracold neutrons is based on their wave properties. Such neutrons can simply be stored in a closed "bank". This idea was put forward by the Soviet physicist Ya. B. Zeldovich in the late 1950s, and the first results were obtained in Dubna at the Institute for Nuclear Research almost a decade later.
Recently, scientists have managed to build a vessel in which ultracold neutrons live until their natural decay.
Free neutrons are able to actively interact with atomic nuclei, causing nuclear reactions. As a result of the interaction of slow neutrons with matter, resonance effects, diffraction scattering in crystals, etc. can be observed. Due to these features, neutrons are widely used in nuclear physics and solid state physics. They play an important role in nuclear power engineering, in the production of transuranium elements and radioactive isotopes, and find practical applications in chemical analysis and geological exploration.
Mesons- hadrons with integer spin The name comes from the Greek word meaning "middle, intermediate", since the masses of the first discovered mesons had intermediate values between the masses of the proton and electron. The baryon charge of mesons is equal to zero. The lightest of the mesons are pions, or pi-mesons p - , p + and p °. Their masses are about 6-7 times less than the mass of a proton. Strange mesons - kaons K + , K - and K ° are more massive. Their masses are almost two times less than the mass of a proton. The characteristic lifetime of these mesons is 10 -8 s.
Almost all hadrons have antiparticles. Thus, a baryon sigma-minus S - has an anti-sigma-plus antiparticle S` + , which is different from S + . The same can be said about other baryons. With mesons, the situation is somewhat different: a negative pion is the antiparticle of a positive pion, and a neutral pion has no antiparticle at all, since it is an antiparticle to itself. At the same time, the neutral kaon K° has an antiparticle K'°. These facts are explained in the quark model of hadrons.
The world of hadrons is huge - it includes more than 350 particles. Most of them are very unstable: they decay into lighter hadrons in the order of 10–23 s. This is the characteristic time of strong interactions; in such a short interval, even light has time to travel a distance equal to only the radius of a proton (10 - 13 cm). It is clear that such short-lived particles cannot leave traces in detectors. Usually their birth is detected by indirect signs. For example, they study the reaction of annihilation of electrons and positrons with the subsequent birth of hadrons. By varying the collision energy of electrons and positrons, it is found that at a certain value of the energy, the yield of hadrons suddenly increases sharply. This fact can be explained by the fact that a particle was born in the intermediate state, the mass of which is equal to the corresponding energy (up to a factor of c 2). This particle instantly decays into other hadrons, and the only trace of its appearance is the peak on the graph of the probability of hadron production versus the collision energy.
Such short-lived particles are called resonances. Most baryons and mesons are resonances. They do not leave "autographs" in cameras and photographs, and yet physicists manage to study their properties: determine mass, lifetime, spin, parity, decay methods, etc.
According to modern concepts, hadrons are not truly elementary particles. They have finite dimensions and a complex structure. Baryons are made up of three quarks. Accordingly, an antibaryon consists of three antiquarks and is always different from a baryon. Mesons are built from a quark and an antiquark. It is clear that mesons, which include pairs of quarks and antiquarks of the same kind, will not have antiparticles. The quarks are kept inside the hadrons by the gluon field. In principle, the theory admits the existence of other hadrons built from a larger number of quarks or, conversely, from a single gluon field. Recently, some experimental data have appeared on the possible existence of such hypothetical particles. The dynamical theory of quarks, which describes their interactions, began to develop relatively recently. Initially, the quark model was proposed to "put things in order" in an overly numerous family of hadrons. This model included quarks of three types, or, as they say, flavors. With the help of quarks, it was possible to bring order to the numerous family of hadrons, distributing them into groups of particles called multiplets. Particles of the same multiplet have close masses, but not only this served as the basis for their classification; in addition to experimental data, in this case, a special mathematical apparatus of group theory was used.
Later it turned out that three quark flavors are not enough to describe all hadrons. In 1974, the so-called psi-mesons were discovered, consisting of a quark and a new type of antiquark (cc¢). This fragrance has been called charm. The new charmed quark c turned out to be much heavier than its "brothers": the lightest of the psi-particles, the J/y meson, has a mass of 3097 MeV, i.e., 3 times heavier than a proton. Its lifetime is about 10 -20 s. A whole family of psi-mesons was discovered with the same quark composition cc¢ , but in excited states and, as a result, having large masses.
Leptons- a group of particles that do not participate in 1 strong interaction (the name comes from the Greek word "leptos" - "light").
All leptons have spin 1/2. Distinguish charged leptons - electron e -, muon m - , heavy lepton t - and the corresponding antiparticles e + , m + and t + and neutral - various kinds of neutrinos.
The first charged lepton was discovered by the electron - in 1897 by the English scientist J. J. Thomson. Its antiparticle, the positron, was found in 1932 in cosmic rays by the American physicist K. Anderson. In 1936, muons were also discovered in the emission of cosmic rays (K. Anderson and S. Neddermeyer). At first, there was a little confusion: they tried to identify muons with a particle, which, according to the theory of the Japanese physicist X. Yukawa, carried strong interactions. It soon became clear, however, that the muon had nothing to do with strong interactions (particles predicted by Yukawa turned out to be n-mesons discovered in 1947). And then the mystery of the muon arose. The fact is that the muon is surprisingly similar to the electron: they have the same electric charge, spin, both
they participate only in weak and electromagnetic interactions, and in a similar way. Their only visible difference lies in their mass: the muon is 206.8 times heavier than the electron (the current value of its mass is m = 105.65943 MeV/c 2 @ 1.88-10 –25 g).
Due to the larger mass, the muon has lost stability, its lifetime is @2.2 10 -6 s.
The electron is stable because it simply has nothing to decay into. Indeed, due to the conservation of electric charge, the decay of an electron would be possible only with the emission of lighter charged particles, but the existence of such particles is still unknown. If the charge conservation law were not a completely accurate law of nature, then the electron could decay, for example, into a neutrino and a photon. The search for such decays, however, was unsuccessful and showed that the lifetime of an electron is at least more than 1022 years (for comparison: our Universe exists "only" about 2 10 -10 years). Therefore, in modern theories, the electron is considered a stable particle. Note, however, that the experimental limits for the lifetime of the proton look even more impressive (at least 1032 years), but theories in which it can decay have recently become very popular.
With the decay of the muon, the situation is simpler, it can decay and actually decays into an electron and a pair of neutrinos of different types: m - ® e - + n e `+ n m . Weak interactions are responsible for this decay. The experimental value of the muon lifetime agrees well with theoretical calculations. Of course, the decay of a positively charged muon occurs in a similar way:
m + ®e + + n e +n m `.
Before they had time to figure out the riddle of the muon, physicists discovered the third charged lepton t (tau - lepton). It was discovered in 1975 in experiments on colliding electron-positron beams at Stanford (USA) by a group of physicists led by M. Pearl during the annihilation of an electron and a positron of very high energies. The heavy tau lepton has a mass nearly 3500 times that of an electron ( m e ~1784 MeV/s 2). It is even almost 2 times heavier than a proton. The lifetime of a t-lepton was measured with sufficient accuracy only in 1981 - 3.4 10 - 13 s. Such a lifetime shows that the weak interactions of t-leptons are very similar to the weak interactions of electrons and muons (it should be borne in mind that the heavier the particle, the faster, under other identical conditions, it decays into lighter ones. The available data suggest that and otherwise the t-lepton is like the electron and the muon.
Charged leptons are united by one more property: in modern theories they are all represented as point objects, which, unlike hadrons, do not have an internal structure. Experiments on the most powerful accelerators at the maximum currently achievable energies show that this is true, at least up to distances @ 10 - 16 cm.
By observing reactions involving leptons, scientists have found that the difference between the number of leptons and antileptons always remains constant. To describe this property, a special quantum number was introduced - the lepton charge L, conditionally assigning the value L= 1 to negatively charged leptons and their accompanying neutrinos, and the value L.= -1 - to their antiparticles. Then this phenomenon is reduced to the law of conservation of lepton charge. Later it was established that the electron and muon neutrinos are not identical to each other, and it was necessary to introduce different, independently conserved lepton charges. Apparently, there is a third type of lepton charge associated with the heavy lepton and its neutrino.
So far, no cases of violation of the law of conservation of lepton charge have been observed. Say, this law forbids neutrinoless muon decays. The ratio of the probabilities of forbidden and ordinary muon decays was estimated in experiments and turned out to be less than 10 -9 -10 -10 . The search for forbidden decays is of great interest, since the possibility of detecting lepton charge nonconservation is not ruled out. It should be emphasized that the lepton charge is not a source of some kind of "lepton" field, but was introduced solely to explain the experimentally observed laws of reactions involving leptons.
The theories that have appeared recently, based on the concept of the unity of the forces of nature, predict the instability of the proton and, at the same time, the violation of the conservation of the lepton charge. What is the reason for the existence of different types of leptons with similar properties and very different masses? What is the nature of lepton charges? And are there any other leptons that are still unknown to us? These questions are currently unanswered. Their solution is connected not only with leptons, but also with other truly elementary particles-quarks, which are the main structural elements of the world of strongly interacting particles. Quarks vary greatly in mass and have their own specific "charges". Pairs of quarks combine together with pairs of leptons (a charged lepton and the corresponding neutrino) into the so-called generations of elementary particles. Many properties of particles are repeated from generation to generation, and the masses of generations differ greatly: the second generation (it includes muons) is heavier than the first (with electrons), and the third generation (including t-leptons) is heavier than the second. Research into many of the mysteries of these generations is just beginning.
Electron is a negatively charged elementary particle, the carrier of the smallest known mass and the smallest electric charge in nature. Opened in 1897 by the English scientist J. J. Thomson.
An electron is a component of an atom, the number of electrons in a neutral atom is equal to the atomic number, i.e. the number of protons in the nucleus.
The first accurate measurements of the electron charge were carried out in 1909-1913. American physicist R. Milliken. The modern value of the absolute value of the elementary charge is
e =(4.803242±0.000014)*10 -10 or approximately 1.6*10 -19 C. it is believed that this charge is indeed "elementary", i.e., it cannot be divided into parts, and the charges of any objects are its integer multiples. Together with the Planck constant H and the speed of light c, the elementary charge forms a dimensionless constant a = e 2 / hc ~ 1/ 137. The fine structure constant a is one of the most important parameters of quantum electrodynamics, it determines the intensity of electromagnetic interactions. Mass of an electron m e \u003d (9.109534 ± 0.000047) * 10 -28 g (in energy units ~ 0.5 MeV / s 2). If the laws of conservation of energy and electric charge are valid, then any decay of an electron is prohibited. Therefore, the electron is stable; experimentally obtained that the time of his life is not less than 1022 years.
In 1925, American physicists S. Goudsmit and J. Uhlenbeck introduced the internal angular momentum of an electron - spin (s) to explain the features of atomic spectra. The spin of an electron is half of Planck's constant (H - 1.055*10 -34 J/s), but physicists usually say simply that the spin of an electron is 1/2:5 = 1/2. The spin of an electron is associated with its own magnetic moment. The magnetic moment of the electron had to be equal to exactly one Bohr magneton.
However, in 1947 it was found in experiments that the magnetic moment is approximately 0.1% larger than the Bohr magneton. The explanation of this fact was given taking into account the vacuum polarization in quantum electrodynamics. Very laborious calculations gave the theoretical value g e = 2*(1.001159652460 ± 0.000000000148), which can be compared with experimental data: for an electron g e = 2-(1.001159652200 ± 0.000000000040) and a positron g e = 2 (1, (10 1159652222 ± 0.000000000050). The values are calculated and measured with an accuracy of up to twelve decimal places, and the accuracy of experimental work is higher
"accuracies of theoretical calculations. These are the most accurate measurements in particle physics.
The features of the motion of electrons in atoms, subject to the equations of quantum mechanics, determine the optical, electrical, magnetic, chemical and mechanical properties of substances.
Electrons participate in electromagnetic, weak and gravitational interactions.
Weak interactions of electrons manifest themselves, for example, in nonconservation processes in atomic spectra or in reactions between electrons and neutrinos.
There is no data on the internal structure of the electron. Modern theories proceed from the concept of leptons as point particles. At present, this has been experimentally verified up to distances of 10 -16 cm. New data may appear only with an increase in the particle collision energy in future accelerators.
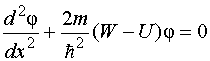 In other words, the probability of finding an electron outside the “box” is zero. The problem of electron motion in a rectangular potential “box” with infinitely high walls is reduced to solving the equation
In other words, the probability of finding an electron outside the “box” is zero. The problem of electron motion in a rectangular potential “box” with infinitely high walls is reduced to solving the equation 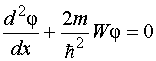 Other meanings W electron energies are impossible: the probability of finding an electron inside the “box” with an energy different from W n, equals zero. Physical quantities that take only certain discrete values are called quantized. Quantized values W n called energy levels, and the numbers n, which determine the energy levels of the electron, - quantum numbers. Thus, an electron in a potential “box” can be at a certain energy level W n. In this case, it is in a certain quantum state n. The energy levels in this case are so closely spaced that they can be considered quasi-continuous. For such a potential “box”, energy quantization gives results that are not so significantly different from the results of classical physics as in the case of an atomic-sized “box”. Calculations show that with an increase in the quantum number n value becomes small compared to W n, i.e. there is a relative convergence of energy levels. For large quantum numbers n energy quantization gives results close to those of the classical treatment. This expresses an important conformity principle, most fully formulated Borom in 1923: for large quantum numbers, the conclusions and results of quantum mechanics must correspond to the classical results. 82) Rutherford's nuclear model. Bohr's postulates From a radioactive source enclosed in a lead container, α-particles were directed to a thin metal foil. Scattered particles hit a screen covered with a layer of zinc sulfide crystals capable of glowing under the impact of fast charged particles. Scintillations (flashes) on the screen were observed with the eye using a microscope. Observations of scattered α-particles in Rutherford's experiment could be carried out at various angles φ to the initial direction of the beam. Most alpha particles were found to pass through a thin layer of metal with little or no deflection. However, a small part of the particles is deflected at significant angles exceeding 30°. Very rare alpha particles (approximately one in ten thousand) were deflected through angles close to 180°. Thus, the experiments of Rutherford and his colleagues led to the conclusion that in the center of the atom there is a dense positively charged nucleus, the diameter of which does not exceed 10–14–10–15 m. This nucleus occupies only 10–12 of the total volume of the atom, but contains the entire positive charge and not less than 99.95% of its mass. The substance constituting the nucleus of an atom should have been assigned a colossal density of the order c ≈ 10 15 g/cm 3 . The charge of the nucleus must be equal to the total charge of all the electrons that make up the atom. Subsequently, it was found that if the charge of the electron is taken as unity, then the charge of the nucleus is exactly equal to the number of this element in the periodic table. Rutherford suggested planetary model of the atom. According to this model, a positively charged nucleus is located in the center of the atom, in which almost the entire mass of the atom is concentrated. The atom as a whole is neutral. Electrons rotate around the nucleus, like planets, under the influence of Coulomb forces from the nucleus (Fig. 6.1.4). Electrons cannot be at rest, as they would fall on the nucleus. Bohr's postulates Bohr's postulates are the basic assumptions formulated by Niels Bohr in 1913 to explain the regularities of the line spectrum of the hydrogen atom and hydrogen-like ions (the Balmer-Rydberg formula) and the quantum nature of the emission and absorption of light. Bohr proceeded from Rutherford's planetary model of the atom. Postulates: 10 An atom can only be in special stationary, or quantum, states, each of which corresponds to a certain energy. In a stationary state, an atom does not radiate electromagnetic waves. 2) An electron in an atom, without losing energy, moves along certain discrete circular orbits, for which the angular momentum is quantized: , where are natural numbers, and is Planck's constant. The stay of an electron in orbit determines the energy of these stationary states. 3) When an electron moves from an orbit (energy level) to an orbit, an energy quantum is emitted or absorbed, where are the energy levels between which the transition is made. When moving from the upper level to the lower one, energy is emitted, and when moving from the lower to the upper one, it is absorbed. Using these postulates and the laws of classical mechanics, Bohr proposed a model of the atom, now called the Bohr model of the atom. Later, Sommerfeld extended Bohr's theory to the case of elliptical orbits. It is called the Bohr-Sommerfeld model. 83) Quantum numbers. Pauli principle. Maximum number of electrons
quantum numbers
- energy parameters that determine the state of the electron and the type of atomic orbital in which it is located. Pauli principle
(exclusion principle) - one of the fundamental principles of quantum mechanics, according to which two or more identical fermions (particles with a half-integer spin) cannot simultaneously be in the same quantum state. The Pauli principle helps explain a variety of physical phenomena. The consequence of the principle is the presence of electron shells in the structure of the atom, which, in turn, implies a variety of chemical elements and their compounds. The number of electrons in a single atom is equal to the number of protons. Since electrons are fermions, the Pauli principle forbids them from taking the same quantum states. As a result, all electrons cannot be in the same quantum state with the lowest energy (for an unexcited atom), but sequentially fill quantum states with the lowest total energy (at the same time, do not forget that electrons are indistinguishable, and it is impossible to say in which quantum state the given electron is located). An example is the unexcited lithium atom (Li), in which two electrons are in the 1S orbital (the lowest in energy), while their own momentum differs and the third electron cannot occupy the 1S orbital, since the Pauli prohibition will be violated. Therefore, the third electron occupies the 2S orbital (the next lowest energy orbital after the 1S). Maximum number of electrons
Other meanings W electron energies are impossible: the probability of finding an electron inside the “box” with an energy different from W n, equals zero. Physical quantities that take only certain discrete values are called quantized. Quantized values W n called energy levels, and the numbers n, which determine the energy levels of the electron, - quantum numbers. Thus, an electron in a potential “box” can be at a certain energy level W n. In this case, it is in a certain quantum state n. The energy levels in this case are so closely spaced that they can be considered quasi-continuous. For such a potential “box”, energy quantization gives results that are not so significantly different from the results of classical physics as in the case of an atomic-sized “box”. Calculations show that with an increase in the quantum number n value becomes small compared to W n, i.e. there is a relative convergence of energy levels. For large quantum numbers n energy quantization gives results close to those of the classical treatment. This expresses an important conformity principle, most fully formulated Borom in 1923: for large quantum numbers, the conclusions and results of quantum mechanics must correspond to the classical results. 82) Rutherford's nuclear model. Bohr's postulates From a radioactive source enclosed in a lead container, α-particles were directed to a thin metal foil. Scattered particles hit a screen covered with a layer of zinc sulfide crystals capable of glowing under the impact of fast charged particles. Scintillations (flashes) on the screen were observed with the eye using a microscope. Observations of scattered α-particles in Rutherford's experiment could be carried out at various angles φ to the initial direction of the beam. Most alpha particles were found to pass through a thin layer of metal with little or no deflection. However, a small part of the particles is deflected at significant angles exceeding 30°. Very rare alpha particles (approximately one in ten thousand) were deflected through angles close to 180°. Thus, the experiments of Rutherford and his colleagues led to the conclusion that in the center of the atom there is a dense positively charged nucleus, the diameter of which does not exceed 10–14–10–15 m. This nucleus occupies only 10–12 of the total volume of the atom, but contains the entire positive charge and not less than 99.95% of its mass. The substance constituting the nucleus of an atom should have been assigned a colossal density of the order c ≈ 10 15 g/cm 3 . The charge of the nucleus must be equal to the total charge of all the electrons that make up the atom. Subsequently, it was found that if the charge of the electron is taken as unity, then the charge of the nucleus is exactly equal to the number of this element in the periodic table. Rutherford suggested planetary model of the atom. According to this model, a positively charged nucleus is located in the center of the atom, in which almost the entire mass of the atom is concentrated. The atom as a whole is neutral. Electrons rotate around the nucleus, like planets, under the influence of Coulomb forces from the nucleus (Fig. 6.1.4). Electrons cannot be at rest, as they would fall on the nucleus. Bohr's postulates Bohr's postulates are the basic assumptions formulated by Niels Bohr in 1913 to explain the regularities of the line spectrum of the hydrogen atom and hydrogen-like ions (the Balmer-Rydberg formula) and the quantum nature of the emission and absorption of light. Bohr proceeded from Rutherford's planetary model of the atom. Postulates: 10 An atom can only be in special stationary, or quantum, states, each of which corresponds to a certain energy. In a stationary state, an atom does not radiate electromagnetic waves. 2) An electron in an atom, without losing energy, moves along certain discrete circular orbits, for which the angular momentum is quantized: , where are natural numbers, and is Planck's constant. The stay of an electron in orbit determines the energy of these stationary states. 3) When an electron moves from an orbit (energy level) to an orbit, an energy quantum is emitted or absorbed, where are the energy levels between which the transition is made. When moving from the upper level to the lower one, energy is emitted, and when moving from the lower to the upper one, it is absorbed. Using these postulates and the laws of classical mechanics, Bohr proposed a model of the atom, now called the Bohr model of the atom. Later, Sommerfeld extended Bohr's theory to the case of elliptical orbits. It is called the Bohr-Sommerfeld model. 83) Quantum numbers. Pauli principle. Maximum number of electrons
quantum numbers
- energy parameters that determine the state of the electron and the type of atomic orbital in which it is located. Pauli principle
(exclusion principle) - one of the fundamental principles of quantum mechanics, according to which two or more identical fermions (particles with a half-integer spin) cannot simultaneously be in the same quantum state. The Pauli principle helps explain a variety of physical phenomena. The consequence of the principle is the presence of electron shells in the structure of the atom, which, in turn, implies a variety of chemical elements and their compounds. The number of electrons in a single atom is equal to the number of protons. Since electrons are fermions, the Pauli principle forbids them from taking the same quantum states. As a result, all electrons cannot be in the same quantum state with the lowest energy (for an unexcited atom), but sequentially fill quantum states with the lowest total energy (at the same time, do not forget that electrons are indistinguishable, and it is impossible to say in which quantum state the given electron is located). An example is the unexcited lithium atom (Li), in which two electrons are in the 1S orbital (the lowest in energy), while their own momentum differs and the third electron cannot occupy the 1S orbital, since the Pauli prohibition will be violated. Therefore, the third electron occupies the 2S orbital (the next lowest energy orbital after the 1S). Maximum number of electrons
Core charge equals Ze, where e is the charge of the proton, Z– charge number equal to serial number chemical element in Mendeleev's periodic system of elements, i.e. the number of protons in the nucleus. The number of neutrons in a nucleus is denoted N. Usually Z > N.
AT the composition of the atomic nucleus includes elementary particles : protons and neutrons (nucleons from the Latin word nucleus - core). Such a proton-neutron model of the nucleus was proposed by the Soviet physicist in 1932 D.D. Ivanenko. The proton has a positive charge e + = 1.06 10 -19 C and a rest mass m p\u003d 1.673 10 -27 kg \u003d 1836 me. Neutron ( n) is a neutral particle with rest mass m n= 1.675 10 -27 kg = 1839 me(where the mass of the electron me, is equal to 0.91 10 -31 kg). On fig. 9.1 shows the structure of the helium atom according to the ideas of the late XX - early XXI century.
Number of nucleons in the nucleus A = Z + N called mass number . nuclei with the same Z, but different BUT called isotopes. Kernels, which, at the same A have different Z, are called isobars.
86 ) The binding energy of the nucleus. mass defect. Ionization potential.
Core binding energy
The binding energy of the nucleus is equal to the minimum energy that must be expended for the complete splitting of the nucleus into individual particles. It follows from the law of conservation of energy that the binding energy is equal to the energy that is released during the formation of a nucleus from individual particles.
The binding energy of any nucleus can be determined by accurately measuring its mass. At present, physicists have learned to measure the masses of particles - electrons, protons, neutrons, nuclei, etc. - with very high accuracy. These measurements show that the mass of any nucleus M i is always less than the sum of the masses of its constituent protons and neutrons:
| M I< Zm p+ Nm n. |
Mass difference
| This energy is released during the formation of the nucleus in the form of radiation of γ-quanta |
| Ionization potential of an atom- minimum potential difference U, to which an electron must pass in an accelerating electric field in order to acquire kinetic energy sufficient to ionize the atom. |
Ionization potential U is closely related to the ionization energy by the relation:
E=Ue,where e-elementary electric charge.
The ionization energy of an atom is an internal property of the particle and does not depend on the ionization method, while the ionization potential can be said to be a characteristic of the historically first ionization method.
87 ) Radioactive radiation. Types of nuclear decays. Law of radioactive decay. Its characteristics.
Currently under radioactivity understand the ability of some atomic nuclei to spontaneously (spontaneously) transform into other nuclei with the emission of various types of radioactive radiation and elementary particles. Radioactivity is divided into natural(observed in unstable isotopes that exist in nature) and artificial(observed in isotopes obtained through nuclear reactions). There is no fundamental difference between these two types of radioactivity, since the laws of radioactive transformation are the same in both cases.
There are three types of radioactive radiation: -, - and -radiation.
Objects of study and tasks of agricultural radiobiology. History of the development of radiobiology.
The fundamental task which is the subject of radiobiology, is the discovery of the general patterns of the biological response to the effects of ionizing radiation, which are the scientific basis for the hygienic regulation of the radiation factor and mastering the art of controlling the body's radiation reactions.
The development of radiobiology is divided into three stages:
Stage 1 The development of radiobiology is characterized by works of a descriptive nature, when scientists try to explain the effects described earlier.
The first scientists who drew attention to the effect of radium on the skin were the Germans G. Walchow and Gisel.
The main discoveries are:
1. Discovery that cell division is inhibited under the action of ionizing radiation.
2. The Bergagnier-Tribondo law (1903-1906): a cell is the more radiosensitive, the more it has the ability to reproduce (divide) and the less clearly expressed their morphology and functions, i.e. than they are less differentiated.
Stage 2 The development of radiobiology is associated with the development of the "dose-effect" theory. On the one hand, it was found that with an increase in the radiation dose, the damaging effect increases.
A significant discovery in 1922 was the theory of ionization events in a sensitive volume.
Stage 3 began in the 1940s and 1950s. Methods of quantitative atomic radiobiology have been greatly developed.
In particular, it was established that from the very beginning, radioactive emissions are not the same, and in 1903 a drawing appeared in the dissertation of Maria Skladowska-Curie.
1998 Becquerel proved that β-rays are a stream of fast electrons charged negatively.
In 1988-1899, Paul Villard established that electromagnetic radiation that does not respond to charge and is similar to X-rays is γ-rays; they are electrically neutral (have no charge), have no rest mass, and consist of individual portions of energy.
In 1899, Ernest Rutherford proved that α-rays are a stream of helium nuclei, positively charged due to protons, which have a positive charge due to a set of quarks.
The first reactor for the production of weapons-grade plutonium was built in 1940-1945.
Origin of natural radioactive atoms.
Eating. radioactive atoms have been around since the Earth appeared.
In 1896, Becquerel discovered that some uranium salts emit penetrating radiation similar to that discovered by Roentgen a year earlier. The enormous significance of this discovery was not immediately understood. A few years later, Pierre and Marie Curie gave further impetus to the study of this new field of physics. They managed to isolate two substances from uranium resin blende, the radioactivity of which is much more intense than the radioactivity of uranium. Rutherford and Soddy, investigating the phenomenon discovered by Becquerel, soon found that the phenomenon of radioactivity can be explained by assuming that the atoms of uranium and radium are not stable, but decay at a characteristic rate for each. In this case, atoms of other elements are formed. Radioactive transformations proceed spontaneously. The speed of their flow is not affected by changes in temperature and pressure, the presence of electric and magnetic fields, the type of chemical compound of a given radioactive element and its state of aggregation. Radioactive decay is a property of the atomic nucleus itself and depends only on its internal state. It is impossible to influence the course of the process of radioactive decay without changing the state of the atomic nucleus. Radioactivity is the ability of some atomic nuclei to spontaneously (spontaneously) decay with the emission of α-, β-, γ-rays, and sometimes other particles.
Radioactive radiation produces an ionization effect. A radioactive element is constantly releasing energy, and therefore its temperature is always slightly higher than the ambient temperature. Already the first studies showed that the radioactivity of an element does not depend on whether it is in its pure form or in the composition of any chemical compounds.
The structure of the atom and the characteristics of elementary particles.
The smallest particle of a chemical element, which is the carrier of its chemical properties - is called atom.
An atom consists of an atomic nucleus and an electron shell.
The nucleus of an atom consists of protons (p+) and neutrons (n0).
The number of protons N(p+) is equal to the charge of the nucleus (Z) and the serial number of the element in the natural series of elements (and in the periodic system of elements).
The sum of the number of neutrons N(n0), denoted simply by the letter N, and the number of protons Z is called the mass number and is denoted by the letter A.
The electron shell of an atom consists of electrons (e-) moving around the nucleus.
The number of electrons N(e-) in the electron shell of a neutral atom is equal to the number of protons Z in its nucleus.
An atom of any element can be divided into subatomic (elementary) particles, but in this case they will not have the properties of an atom.
In a free state, elementary particles are characterized by mass, electric charge and their own rotation (spin).
Elementary particles are divided into classes:
1. Photons (quanta) are quanta of the electro-magnetic field. They have no electrical charge and no rest mass.
2. Leptons("lungs"). These include: electrons (e -); positrons (e +) - these are the antiparticles of the electron, they exist in the nucleus, when they enter the nucleus and meet in the electron, annihilation occurs, i.e. mutual destruction; muons (µ–, µ+) – smaller particles, can have a positive and negative charge; tau leptons (t - , t +); neutrinos and antineutrinos - the last two particles do not have an electric charge., differ in spin (motion).
3. Mesons("medium") - unstable particles. π mesons can have a positive, negative and neutral charge and exist in motion and in the form of material particles (the mass of material particles is about 270 times greater than the mass of an electron). K-mesons have a positive and negative charge, their mass is 970 times the mass of an electron. The lifetime is very short (10–8 sec), they are not stable and decay with the formation of π mesons and leptons or only leptons. Eta mesons (η) - 1074 times heavier than an electron, lifetime 10 -19 sec, decay into π mesons and photons.
4. baryon class brings together protons , neutrons , antiprotons , antineutrons , and unstable, whose mass is greater than the mass of nucleons - they are called hyperons . With the exception of the proton and antiproton, all baryons are unstable. When a baryon decays, a baryon (usually a proton) is necessarily formed.
In addition to these, a large number of short-lived particles were found - resonances.
Fanatical mathematicians, who love to count everything in the world, have long wanted to know the answer to the fundamental question: how many particles are there in the universe? Considering that approximately 5 trillion hydrogen atoms can fit on the head of a pin alone, and each of them consists of 4 elementary particles (1 electron and 3 quarks in a proton), it can be safely assumed that the number of particles in the observable universe is beyond human representation.
Anyway, physics professor Tony Padilla from the University of Nottingham has developed a way to estimate the total number of particles in the universe without taking into account photons or neutrinos, since they have no (or rather, almost no) mass:
For his calculations, the scientist used data obtained with the Planck telescope, which was used to measure the CMB, which is the oldest visible light in the universe and thus forms a semblance of its boundary. Thanks to the telescope, scientists were able to estimate the density and radius of the visible universe.
Another necessary variable is the fraction of matter contained in the baryons. These particles are made up of three quarks, and the best-known baryons today are protons and neutrons, and therefore Padilla considers them in his example. Finally, for the calculation it is necessary to know the masses of the proton and neutron (which approximately coincide with each other), after which you can proceed to the calculations.
What does a physicist do? He takes the density of the visible universe, multiplies it by a fraction of the density of the baryons alone, and then multiplies the result by the volume of the universe. He divides the resulting mass of all baryons in the Universe by the mass of one baryon and obtains the total number of baryons. But we are not interested in baryons, our goal is elementary particles.
It is known that each baryon consists of three quarks - they are exactly what we need. Moreover, the total number of protons (as we all know from the school chemistry course) is equal to the total number of electrons, which are also elementary particles. In addition, astronomers have found that 75% of the matter in the universe is hydrogen, and the remaining 25% is helium, while other elements can be neglected in calculations of this scale. Padilla calculates the number of neutrons, protons and electrons, and then multiplies the first two positions by three - and we finally have the final result.
3.28x10 80. More than three vigintillion.
328.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.000.
The most interesting thing is that, given the scale of the universe, these particles do not fill even a large part of its total volume. As a result, there is only one (!) elementary particle per cubic meter of the Universe.






