Types of chemical reactions in organic chemistry examples. Types of chemical reactions in organic chemistry lesson plan in chemistry (grade 10) on the topic. Classification of organic reactions by mechanism. Examples
Many substitution reactions open the way to the production of a variety of compounds that have economic applications. Electrophilic and nucleophilic substitution plays a huge role in chemical science and industry. In organic synthesis, these processes have a number of features that should be paid attention to.
Variety of chemical phenomena. Substitution reactions
Chemical changes associated with the transformation of substances are distinguished by a number of features. The final results and thermal effects may vary; Some processes go to completion, in others a change in substances occurs, often accompanied by an increase or decrease in the degree of oxidation. When classifying chemical phenomena according to their final result, attention is paid to the qualitative and quantitative differences between reagents and products. Based on these characteristics, 7 types of chemical transformations can be distinguished, including substitution, which follows the scheme: A-B + C A-C + B. A simplified notation of a whole class of chemical phenomena gives the idea that among the starting substances there is a so-called “attack "a particle that replaces an atom, ion, or functional group in a reagent. The substitution reaction is characteristic of limiting and
Substitution reactions can occur in the form of a double exchange: A-B + C-E A-C + B-E. One of the subspecies is the displacement, for example, of copper with iron from a solution of copper sulfate: CuSO 4 + Fe = FeSO 4 + Cu. The “attacking” particle can be atoms, ions or functional groups

Homolytic substitution (radical, SR)
With the radical mechanism of breaking covalent bonds, an electron pair common to different elements is proportionally distributed between the “fragments” of the molecule. Free radicals are formed. These are unstable particles, the stabilization of which occurs as a result of subsequent transformations. For example, when producing ethane from methane, free radicals appear that participate in the substitution reaction: CH 4 CH 3. + .N; CH 3. + .CH 3 → C2H5; N. + .N → N2. Homolytic bond cleavage according to the above substitution mechanism is of a chain nature. In methane, the H atoms can be successively replaced by chlorine. The reaction with bromine occurs similarly, but iodine is unable to directly replace hydrogen in alkanes; fluorine reacts with them too vigorously.

Heterolytic bond breaking method
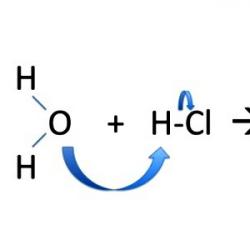
With the ionic mechanism of substitution reactions, electrons are unevenly distributed between newly formed particles. The bonding pair of electrons goes entirely to one of the “fragments”, most often to the bond partner towards which the negative density in the polar molecule was shifted. Substitution reactions include the formation of methyl alcohol CH 3 OH. In bromomethane CH3Br, the cleavage of the molecule is heterolytic, and the charged particles are stable. Methyl acquires a positive charge, and bromine acquires a negative charge: CH 3 Br → CH 3 + + Br - ; NaOH → Na + + OH - ; CH 3 + + OH - → CH 3 OH; Na + + Br - ↔ NaBr.

Electrophiles and nucleophiles
Particles that lack electrons and can accept them are called “electrophiles.” These include carbon atoms connected to halogens in haloalkanes. Nucleophiles have increased electron density; they “donate” a pair of electrons when creating a covalent bond. In substitution reactions, nucleophiles rich in negative charges are attacked by electron-starved electrophiles. This phenomenon is associated with the movement of an atom or other particle - a leaving group. Another type of substitution reaction is the attack of an electrophile by a nucleophile. It is sometimes difficult to distinguish between two processes and to attribute substitution to one type or another, since it is difficult to accurately indicate which of the molecules is the substrate and which is the reagent. Typically in such cases the following factors are taken into account:
- the nature of the leaving group;
- nucleophile reactivity;
- nature of the solvent;
- structure of the alkyl part.
Nucleophilic substitution (SN)
During the interaction process in an organic molecule, an increase in polarization is observed. In equations, a partial positive or negative charge is indicated by a letter of the Greek alphabet. Bond polarization makes it possible to judge the nature of its rupture and the further behavior of the “fragments” of the molecule. For example, the carbon atom in iodomethane has a partial positive charge and is an electrophilic center. It attracts that part of the water dipole where oxygen, which has an excess of electrons, is located. When an electrophile interacts with a nucleophilic reagent, methanol is formed: CH 3 I + H 2 O → CH 3 OH + HI. Nucleophilic substitution reactions take place with the participation of a negatively charged ion or molecule with a free electron pair that is not involved in the creation of a chemical bond. The active participation of iodomethane in SN 2 reactions is explained by its openness to nucleophilic attack and the mobility of iodine.

Electrophilic substitution (SE)
An organic molecule may contain a nucleophilic center, which is characterized by an excess of electron density. It reacts with an electrophilic reagent lacking negative charges. Such particles include atoms with free orbitals and molecules with areas of low electron density. B carbon, which has a “-” charge, interacts with the positive part of the water dipole - with hydrogen: CH 3 Na + H 2 O → CH 4 + NaOH. The product of this electrophilic substitution reaction is methane. In heterolytic reactions, oppositely charged centers of organic molecules interact, which makes them similar to ions in the chemistry of inorganic substances. It should not be overlooked that the transformation of organic compounds is rarely accompanied by the formation of true cations and anions.

Monomolecular and bimolecular reactions
Nucleophilic substitution is monomolecular (SN1). This mechanism is used to hydrolyze an important product of organic synthesis—tertiary butyl chloride. The first stage is slow; it is associated with gradual dissociation into carbonium cation and chloride anion. The second stage proceeds faster, the reaction of carbonium ion with water occurs. replacing the halogen in the alkane with an hydroxy group and obtaining a primary alcohol: (CH 3) 3 C—Cl → (CH 3) 3 C + + Cl - ; (CH 3) 3 C + + H 2 O → (CH 3) 3 C—OH + H + . The one-stage hydrolysis of primary and secondary alkyl halides is characterized by the simultaneous destruction of the carbon-halogen bond and the formation of a C-OH pair. This is a nucleophilic bimolecular substitution (SN2) mechanism.
Mechanism of heterolytic replacement
The substitution mechanism is associated with electron transfer and the creation of intermediate complexes. The faster the reaction occurs, the easier its characteristic intermediate products arise. Often the process goes in several directions simultaneously. The advantage usually goes to the path that uses particles that require the least amount of energy for their formation. For example, the presence of a double bond increases the probability of the appearance of an allylic cation CH2=CH—CH 2 + compared to the CH 3 + ion. The reason lies in the electron density of the multiple bond, which affects the delocalization of the positive charge dispersed throughout the molecule.
Benzene substitution reactions
The group characterized by electrophilic substitution is arenes. The benzene ring is a convenient target for electrophilic attack. The process begins with bond polarization in the second reagent, resulting in the formation of an electrophile adjacent to the electron cloud of the benzene ring. As a result, a transition complex appears. There is not yet a full connection between the electrophilic particle and one of the carbon atoms; it is attracted to the entire negative charge of the “aromatic six” electrons. In the third stage of the process, the electrophile and one carbon atom of the ring are linked by a shared pair of electrons (covalent bond). But in this case, the “aromatic six” is destroyed, which is unfavorable from the point of view of achieving a stable, stable energy state. A phenomenon that can be called “proton ejection” is observed. H+ is eliminated, and a stable communication system characteristic of arenes is restored. The by-product contains a hydrogen cation from the benzene ring and an anion from the second reagent.

Examples of substitution reactions from organic chemistry
Alkanes are especially characterized by a substitution reaction. Examples of electrophilic and nucleophilic transformations can be given for cycloalkanes and arenes. Similar reactions in molecules of organic substances occur under normal conditions, but more often when heated and in the presence of catalysts. Common and well-studied processes include electrophilic substitution in the aromatic ring. The most important reactions of this type:
- Nitration of benzene in the presence of H 2 SO 4 proceeds according to the scheme: C 6 H 6 → C 6 H 5 -NO 2.
- Catalytic halogenation of benzene, in particular chlorination, according to the equation: C 6 H 6 + Cl 2 → C 6 H 5 Cl + HCl.
- The aromatic process proceeds with “fuming” sulfuric acid, benzenesulfonic acids are formed.
- Alkylation is the replacement of a hydrogen atom from the benzene ring with an alkyl.
- Acylation—formation of ketones.
- Formylation is the replacement of hydrogen with a CHO group and the formation of aldehydes.
Substitution reactions include reactions in alkanes and cycloalkanes in which halogens attack an accessible C-H bond. The formation of derivatives may involve the replacement of one, two or all hydrogen atoms in saturated hydrocarbons and cycloparaffins. Many of the haloalkanes with small molecular weights are used in the production of more complex substances belonging to different classes. The progress achieved in studying the mechanisms of substitution reactions has given a powerful impetus to the development of syntheses based on alkanes, cycloparaffins, arenes and halogenated hydrocarbons.
CH 3 -CH 3 + Cl 2 – (hv) ---- CH 3 -CH 2 Cl + HCl
C 6 H 5 CH 3 + Cl 2 --- 500 C --- C 6 H 5 CH 2 Cl + HCl
Addition reactions
Such reactions are typical for organic compounds containing multiple (double or triple) bonds. Reactions of this type include reactions of addition of halogens, hydrogen halides and water to alkenes and alkynes
CH 3 -CH=CH 2 + HCl ---- CH 3 -CH(Cl)-CH 3
Elimination reactions
These are reactions that lead to the formation of multiple bonds. When eliminating hydrogen halides and water, a certain selectivity of the reaction is observed, described by Zaitsev's rule, according to which a hydrogen atom is eliminated from the carbon atom at which there are fewer hydrogen atoms. Example reaction
CH3-CH(Cl)-CH 2 -CH 3 + KOH →CH 3 -CH=CH-CH 3 + HCl
Polymerization and polycondensation
n(CH 2 =CHCl) (-CH 2 -CHCl)n
Redox
The most intense of the oxidative reactions is combustion, a reaction characteristic of all classes of organic compounds. In this case, depending on the combustion conditions, carbon is oxidized to C (soot), CO or CO 2, and hydrogen is converted into water. However, for organic chemists, oxidation reactions carried out under much milder conditions than combustion are of great interest. Oxidizing agents used: solutions of Br2 in water or Cl2 in CCl 4 ; KMnO 4 in water or dilute acid; copper oxide; freshly precipitated silver(I) or copper(II) hydroxides.
3C 2 H 2 + 8KMnO 4 +4H 2 O→3HOOC-COOH + 8MnO 2 + 8KOH
Esterification (and its reverse hydrolysis reaction)
R 1 COOH + HOR 2 H+ R 1 COOR 2 + H 2 O
Cycloaddition
Y R Y-R
‖ + ‖ → ǀ ǀ
R Y R-Y
‖ + →
11. Classification of organic reactions by mechanism. Examples.
The reaction mechanism involves a detailed step-by-step description of chemical reactions. At the same time, it is established which covalent bonds are broken, in what order and in what way. The formation of new bonds during the reaction process is also carefully described. When considering the reaction mechanism, first of all, pay attention to the method of breaking the covalent bond in the reacting molecule. There are two such ways - homolytic and heterolytic.
Radical reactions proceed by homolytic (radical) cleavage of a covalent bond:

Non-polar or low-polar covalent bonds (C–C, N–N, C–H) undergo radical cleavage at high temperatures or under the influence of light. The carbon in the CH 3 radical has 7 outer electrons (instead of a stable octet shell in CH 4). Radicals are unstable; they tend to capture the missing electron (up to a pair or up to an octet). One of the ways to form stable products is dimerization (the combination of two radicals):
CH 3 + CH 3 CH 3 : CH 3,
N + N N : N.
Radical reactions - these are, for example, reactions of chlorination, bromination and nitration of alkanes:

Ionic reactions occur with heterolytic bond cleavage. In this case, short-lived organic ions - carbocations and carbanions - with a charge on the carbon atom are intermediately formed. In ionic reactions, the bonding electron pair is not separated, but passes entirely to one of the atoms, turning it into an anion:

Strongly polar (H–O, C–O) and easily polarizable (C–Br, C–I) bonds are prone to heterolytic cleavage.
Distinguish nucleophilic reactions (nucleophile– looking for the nucleus, a place with a lack of electrons) and electrophilic reactions (electrophile– looking for electrons). The statement that a particular reaction is nucleophilic or electrophilic always refers to the reagent. Reagent– a substance participating in the reaction with a simpler structure. Substrate– a starting substance with a more complex structure. Outgoing group is a replaceable ion that has been bonded to carbon. Reaction product– new carbon-containing substance (written on the right side of the reaction equation).
TO nucleophilic reagents(nucleophiles) include negatively charged ions, compounds with lone pairs of electrons, compounds with double carbon-carbon bonds. TO electrophilic reagents(electrophiles) include positively charged ions, compounds with unfilled electron shells (AlCl 3, BF 3, FeCl 3), compounds with carbonyl groups, halogens. Electrophiles are any atom, molecule or ion capable of adding a pair of electrons in the process of forming a new bond. The driving force of ionic reactions is the interaction of oppositely charged ions or fragments of different molecules with a partial charge (+ and –).
Examples of different types of ionic reactions.
Nucleophilic substitution :
Electrophilic substitution :
Nucleophilic addition (CN – is added first, then H +):

Electrophilic connection (H + is added first, then X –):

Elimination by the action of nucleophiles (bases) :
Elimination upon action electrophiles (acids) :
Organic reactions can be classified into two general types.
Hemolytic reactions. These reactions proceed by a radical mechanism. We'll look at them in more detail in the next chapter. The kinetics and mechanism of reactions of this type were discussed in Chap. 9.
Heterolytic reactions. These reactions are essentially ionic reactions. They can, in turn, be divided into substitution, addition and elimination reactions.
Substitution reactions
In these reactions, an atom or group of atoms is replaced by another atom or group. As an example of reactions of this type, we give the hydrolysis of chloromethane with the formation of methanol:
The hydroxyl ion is a nucleophile. Therefore, the substitution in question is called nucleophilic substitution. It is designated by the symbol SN. The replaced particle (in this case, a chlorine ion) is called a leaving group.
If we denote the nucleophile by the symbol and the leaving group by the symbol, then we can write the generalized equation for the reaction of nucleophilic substitution at a saturated carbon atom in the alkyl group R as follows:
A study of the rate of reactions of this type shows that reactions can be divided into
Reactions of the type For some reactions of the SN type, the kinetic equation for the reaction rate (see Section 9.1) has the form
Thus, these reactions are first order in the substrate but zero order in the reactant. The kinetics characteristic of a first order reaction is a reliable indication that the rate-limiting step of the reaction is a unimolecular process. Therefore, reactions of this type are indicated by the symbol.
The reaction has zero order with respect to the reagent since its rate does not depend on the concentration of the reagent. Therefore, we can write:
Since the nucleophile does not participate in the rate-limiting step of the reaction, the mechanism of such a reaction must include at least two steps. The following mechanism has been proposed for such reactions:

The first stage is ionization with the formation of a carbocation. This stage is limiting (slow).
An example of this type of reaction is the alkaline hydrolysis of tertiary alkyl halides. For example
In the case under consideration, the reaction rate is determined by the equation
Reactions of the type For some reactions of nucleophilic substitution SN the rate equation has the form
In this case, the reaction is first order in the nucleophile and first order in . In general, it is a second order reaction. This is sufficient reason to believe that the rate-limiting stage of this reaction is a bimolecular process. Therefore, the reaction of the type under consideration is denoted by the symbol Since both the nucleophile and the substrate simultaneously participate in the rate-limiting stage of the reaction, we can think that this reaction proceeds in one stage through a transition state (see Section 9.2):
Hydrolysis of primary alkyl halides in an alkaline medium proceeds according to the mechanism

This reaction has the following kinetic equation:
So far we have considered nucleophilic substitution only at the saturated carbon atom. Nucleophilic substitution is also possible at an unsaturated carbon atom:

Reactions of this type are called nucleophilic acyl substitution.
Electrophilic substitution. Electrophilic substitution reactions can also occur on benzene rings. In this type of substitution, the benzene ring supplies the electrophile with two of its delocalized -electrons. In this case, an intermediate compound is formed - an unstable complex of an electrophile and a leaving group. For a schematic representation of such complexes, an open circle is used, indicating the loss of two -electrons:

An example of electrophilic substitution reactions is the nitration of benzene:

Nitration of benzene is carried out in an installation with a reflux condenser at a temperature of 55 to 60 ° C using a nitrating mixture. This mixture contains equal amounts of concentrated nitric and sulfuric acids. The reaction between these acids leads to the formation of a nitroyl cation
Addition reactions
In reactions of this type, an electrophile or nucleophile is added to an unsaturated carbon atom. We will consider here one example each of electrophilic addition and nucleophilic addition.
An example of electrophilic addition is the reaction between hydrogen bromide and an alkene. To obtain hydrogen bromide in the laboratory, a reaction between concentrated sulfuric acid and sodium bromide can be used (see Section 16.2). Hydrogen bromide molecules are polar because the bromine atom has a negative inductive effect on hydrogen. Therefore, the hydrogen bromide molecule has the properties of a strong acid. According to modern views, the reaction of hydrogen bromide with alkenes occurs in two stages. In the first stage, a positively charged hydrogen atom attacks the double bond, which acts as a source of electrons. As a result, an activated complex and a bromide ion are formed:

The bromide ion then attacks this complex, resulting in the formation of an alkyl bromide:

An example of nucleophilic addition is the addition of hydrogen cyanide to any aldehyde or ketone. First, the aldehyde or ketone is treated with an aqueous solution of sodium cyanide. Then an excess amount of any mineral acid is added, which leads to the formation of hydrogen cyanide HCN. The cyanide ion is a nucleophile. It attacks the positively charged carbon atom on the carbonyl group of the aldehyde or ketone. The positive charge and polarity of the carbonyl group is due to the mesomeric effect, which was described above. The reaction can be represented by the following diagram:

Elimination reactions
These reactions are the reverse of addition reactions. They lead to the removal of any atoms or groups of atoms from two carbon atoms connected to each other by a simple covalent bond, resulting in the formation of a multiple bond between them.
An example of such a reaction is the elimination of hydrogen and halogen from alkyl halides:
To carry out this reaction, the alkyl halide is treated with potassium hydroxide in alcohol at a temperature of 60 °C.
It should be noted that treatment of an alkyl halide with hydroxide also leads to nucleophilic substitution (see above). As a result, two competing substitution and elimination reactions occur simultaneously, which leads to the formation of a mixture of substitution and elimination products. Which of these reactions will be predominant depends on a number of factors, including the environment in which the reaction is carried out. Nucleophilic substitution of alkyl halides is carried out in the presence of water. In contrast, elimination reactions are carried out in the absence of water and at higher temperatures.
So let's say it again!
1. During hemolytic cleavage of a bond, two shared electrons are distributed evenly between atoms.
2. During heterolytic bond cleavage, two shared electrons are distributed unevenly between atoms.
3. A carbanion is an ion containing a carbon atom with a negative charge.
4. A carbocation is an ion containing a carbon atom with a positive charge.
5. Solvent effects can have a significant impact on chemical processes and their equilibrium constants.
6. The effect of the chemical environment of a functional group within a molecule on the reactivity of that functional group is called the structural effect.
7. Electronic effects and steric effects are collectively called structural effects.
8. The two most important electronic effects are the inductive effect and the mesomeric (resonance) effect.
9. The inductive effect is the shift of electron density from one atom to another, which leads to polarization of the bond between the two atoms. This effect can be positive or negative.
10. Molecular particles with multiple bonds can exist in the form of resonant hybrids between two or more resonant structures.
11. The mesomeric (resonance) effect consists in the stabilization of resonant hybrids due to the delocalization of -electrons.
12. Steric hindrance can occur when bulky groups in a molecule mechanically impede the reaction.
13. Nucleophile is a particle that attacks a carbon atom, supplying it with its electron pair. The nucleophile is a Lewis base.
14. An electrophile is a particle that attacks a carbon atom, accepting its electron pair. The nucleophile is a Lewis acid.
15. Hemolytic reactions are radical reactions.
16. Heterolytic reactions are mainly ionic reactions.
17. The replacement of any group in a molecule with a nucleophilic reagent is called nucleophilic substitution. The group being replaced in this case is called the leaving group.
18. Electrophilic substitution on a benzene ring involves the donation of two delocalized electrons to some electrophile.
19. In electrophilic addition reactions, an electrophile is added to an unsaturated carbon atom.
20. The addition of hydrogen cyanide to aldehydes or ketones is an example of nucleophilic addition.
21. In elimination (elimination) reactions, some atoms or groups of atoms are separated from two carbon atoms connected to each other by a simple covalent bond. As a result, a multiple bond is formed between these carbon atoms.

Theory of substitution in aromatic compounds. Electrophilic substitution reactions. Orientants of the 2nd kind (meta-orientants).
Substituents that have a negative inductive effect or negative both inductive and mesomeric effects direct electrophilic substitution to the meta position of the benzene ring and are called orientants of the second kind.
Organic reactions, like inorganic ones, are divided into 3 main types:
1) substitution reaction: CH 4 + CI 2 → CH 3 CI + HCI;
2) elimination reaction: CH 3 CH 2 Br → CH 2 = CH 2 + HBr;
3) addition reaction: CH 2 = CH 2 + HBr → CH 3 CH 2 Br.( polymerization reactions)
Classify by the mechanism of breaking covalent bonds in reacting molecules.
Two ways to break covalent bonds.
1. If a common electron pair is shared between atoms, forming radicals. Radicals-particles with unpaired electrons. This disconnection is called radical (homolytic).Peculiarity This connection is that the radicals that are formed interact with the molecules present in the reaction system or with each other.
The resulting radicals interact with molecules present in the reaction system or with each other: CH 3 + CI 2 → CH 3 CI + CI.
According to the radical mechanism, reactions occur in which bonds of low polarity (C-C, C-H, N-N) are broken at high temperatures, under the influence of light or radioactive radiation.
2. If, when a bond is broken, a common electron pair remains with one atom, then ions – cation and anion. This mechanism is called ionic or heterolytic. It leads to the formation of organic cations or anions: 1) methyl chloride forms a methyl cation and a chloride anion; 2) methyl lithium forms lithium cation and methyl anion.
Organic ions undergo further transformations. In this case, cations interact with nucleophilic(“nucleus-loving”) particles, and organic anions – with electrophilic(“electron-loving”) particles (metal cations, halogens, etc.).
The ionic mechanism is observed when a polar covalent bond is broken (carbon - halogen, carbon - oxygen, etc.).
Organic ionic particles are similar to ions in inorganic chemistry - they have corresponding charges. However, they are sharply different: ions of inorganic compounds are constantly present in aqueous solutions, and organic ionic particles appear only at the moment of reaction.
Therefore, in many cases it is necessary to talk not about free organic ions, but about highly polarized molecules.
The radical mechanism is observed when a non-polar or low-polar covalent bond (carbon-carbon, carbon-hydrogen, etc.) is broken.
Organic ionic particles are similar to ions in inorganic chemistry - they have corresponding charges.