Energy conversion in chloroplasts. The Amazing Energy of Excited Chlorophyll Energy Conversion in Photosynthetic Reaction Centers
The structure of chlorophyll is very well adapted to serve as an intermediary in photochemical processes during photosynthesis. Chlorophyll is good sensitizer- is easily excited by the absorption of light and has the ability to transfer energy (serve as an energy donor) to other molecules (energy acceptors).
In the porphyrin core, the chlorophyll molecule alternates. This system of 18 conjugated double bonds serves as the main chromophore and is responsible for the selective absorption of light energy.
The lifetime of the excited state of chlorophyll molecules can be 10 -8 s. The most stable states of atoms are those in which the valence electrons occupy the lowest energy levels and are distributed over them according to the Pauli principle, i.e. the total spin of all electrons of the atom is 0. This state is called the ground state singlet(S= 0).
If the number of electrons in an atom is even, but the spins of two electrons are parallel, then the total spin is 1 ( S= 1), such a state is called triplet. Plays a major role in the light reactions of photosynthesis. singlet excited state.
If, upon absorption of a light quantum, the electron spins remain antiparallel, the chlorophyll molecule goes into a singlet excited state ( S 1 or S 2). Singlet excited state S 2 is very unstable, the electron quickly (within 10 -12 s) loses some of the energy in the form of heat and goes to the lower level ( S 1), where it can remain for 10 -9 - 10 -8 s. The return of the chlorophyll molecule to its original state can occur in several ways.
Firstly, having given up part of the energy in the form of heat and emitting a quantum of light, the molecule can go to the ground state ( S 0). This phenomenon is called fluorescence. The fluorescence wavelength is longer than the corresponding absorption wavelength.
Secondly, in a singlet excited state S 1, a change in the sign of the electron spin may occur, and the chlorophyll molecule becomes metastable triplet state(T), which has a much longer lifetime - about 10 -5 - 10 -3 s. According to the Pauli principle, there cannot be two electrons with the same spins at the same energy level. This prevents an excited electron in a triplet state from occupying an electron “hole” at the ground energy level ( S 0) until the spin sign changes.
From the triplet state, the molecule can return to the ground energy state, emitting a light quantum of longer wavelengths than during fluorescence. This glow is called phosphorescence.
Third, The energy of the singlet excited state of the chlorophyll molecule can be used during photosynthesis in photochemical reactions and transformed into the energy of chemical bonds of organic compounds.
The extremely interesting and not yet fully understood process of energy production by green plants today appears as follows. A quantum of light, absorbed by a chlorophyll molecule, imparts energy to its electrons, which move to excited levels. From there they travel through other molecules linked to chlorophyll into a single chain of energy generation. If there were no such “commonwealth”, then electrons raised to high energy levels would simply fall to their previous places, and the absorbed energy would dissipate. In other words, the molecule would emit a quantum of energy without performing any chemical work. What would happen is roughly the same thing that happens when a steel ball bounces. He falls, having done almost no work, except perhaps to overcome air friction and hit the ground. It would be a different matter if the ball, having jumped up, for example, closed an electrical circuit, thereby causing the light bulb to light up. Here, too, some energy would be lost, but useful work would be done, although the ball would eventually return to its original state.
Something similar happens with the excited electrons of the chlorophyll molecule. Having used up the excess energy imparted to them by the quantum of light, they return to their previous levels. To whom do the excited electrons transfer their energy? Our good friends - cytochromes, which produce the main energy currency of the body - ATP. Note that the photosynthetic relay race of light quantum energy transfer occurs with a very high efficiency, approximately 97%, and the entire process of photosynthesis performs useful work in slightly less than 30%.
It’s not for nothing that we cited these numbers. The production of ATP by the cell is amazingly perfect. Per unit mass; a living being produces much more energy than the Sun. It is curious that a person weighing 70 kg produces up to 75 kg of ATP per day, that is, more than his own weight! The same amount of ATP produced by industry for technical needs costs no less than 150 thousand dollars.
Energy production is, so to speak, one of the aspects of chlorophyll activity that does not go beyond the body. The other side is more impressive, characterized by the initial and final products of photosynthesis. As a result of this process, organic compounds and oxygen are formed from carbon dioxide and water under the influence of light. Thanks to chlorophyll, 200 billion tons of carbon dioxide are assimilated annually on Earth, which produces 100 billion tons of organic substances and about 145 billion tons of free oxygen.
Today it is generally accepted that thanks to photosynthesis of the first green organisms that appeared about three billion years ago, the modern atmosphere was formed and the conditions for the formation of the biosphere appeared (we have already discussed this above). These are the miracles magnesium works in the porphyrin ring
- synthesis of organic substances from carbon dioxide and water with the obligatory use of light energy:
6CO 2 + 6H 2 O + Q light → C 6 H 12 O 6 + 6O 2.
In higher plants, the organ of photosynthesis is the leaf, the organelles of photosynthesis are chloroplasts (the structure of chloroplasts is lecture No. 7). The membranes of chloroplast thylakoids contain photosynthetic pigments: chlorophylls and carotenoids. There are several different types of chlorophyll ( a, b, c, d), the main one is chlorophyll a. In the chlorophyll molecule, a porphyrin “head” with a magnesium atom in the center and a phytol “tail” can be distinguished. The porphyrin “head” is a flat structure, is hydrophilic and therefore lies on the surface of the membrane that faces the aqueous environment of the stroma. The phytol “tail” is hydrophobic and due to this retains the chlorophyll molecule in the membrane.
Chlorophylls absorb red and blue-violet light, reflect green light and therefore give plants their characteristic green color. Chlorophyll molecules in thylakoid membranes are organized into photosystems. Plants and blue-green algae have photosystem-1 and photosystem-2, while photosynthetic bacteria have photosystem-1. Only photosystem-2 can decompose water to release oxygen and take electrons from the hydrogen of water.
Photosynthesis is a complex multi-step process; photosynthesis reactions are divided into two groups: reactions light phase and reactions dark phase.
Light phase
This phase occurs only in the presence of light in thylakoid membranes with the participation of chlorophyll, electron transport proteins and the enzyme ATP synthetase. Under the influence of a quantum of light, chlorophyll electrons are excited, leave the molecule and enter the outer side of the thylakoid membrane, which ultimately becomes negatively charged. Oxidized chlorophyll molecules are reduced, taking electrons from water located in the intrathylakoid space. This leads to the breakdown or photolysis of water:
H 2 O + Q light → H + + OH - .
Hydroxyl ions give up their electrons, becoming reactive radicals.OH:
OH - → .OH + e - .
OH radicals combine to form water and free oxygen:
4NO. → 2H 2 O + O 2.
In this case, oxygen is removed to the external environment, and protons accumulate inside the thylakoid in the “proton reservoir”. As a result, the thylakoid membrane, on the one hand, is charged positively due to H +, and on the other, due to electrons, it is charged negatively. When the potential difference between the outer and inner sides of the thylakoid membrane reaches 200 mV, protons are pushed through the ATP synthetase channels and ADP is phosphorylated to ATP; Atomic hydrogen is used to restore the specific carrier NADP + (nicotinamide adenine dinucleotide phosphate) to NADPH 2:
2H + + 2e - + NADP → NADPH 2.
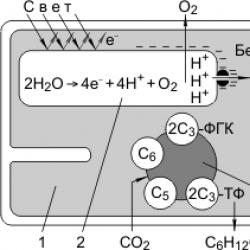
Thus, in the light phase, photolysis of water occurs, which is accompanied by three important processes: 1) ATP synthesis; 2) the formation of NADPH 2; 3) the formation of oxygen. Oxygen diffuses into the atmosphere, ATP and NADPH 2 are transported into the stroma of the chloroplast and participate in the processes of the dark phase.

1 - chloroplast stroma; 2 - grana thylakoid.
Dark phase
This phase occurs in the stroma of the chloroplast. Its reactions do not require light energy, so they occur not only in the light, but also in the dark. Dark phase reactions are a chain of successive transformations of carbon dioxide (coming from the air), leading to the formation of glucose and other organic substances.
The first reaction in this chain is the fixation of carbon dioxide; The carbon dioxide acceptor is a five-carbon sugar. ribulose biphosphate(RiBF); enzyme catalyzes the reaction Ribulose biphosphate carboxylase(RiBP carboxylase). As a result of carboxylation of ribulose bisphosphate, an unstable six-carbon compound is formed, which immediately breaks down into two molecules phosphoglyceric acid(FGK). A cycle of reactions then occurs in which phosphoglyceric acid is converted through a series of intermediates to glucose. These reactions use the energy of ATP and NADPH 2 formed in the light phase; The cycle of these reactions is called the “Calvin cycle”:
6CO 2 + 24H + + ATP → C 6 H 12 O 6 + 6H 2 O.
In addition to glucose, other monomers of complex organic compounds are formed during photosynthesis - amino acids, glycerol and fatty acids, nucleotides. Currently, there are two types of photosynthesis: C 3 - and C 4 photosynthesis.
C 3-photosynthesis

This is a type of photosynthesis in which the first product is three-carbon (C3) compounds. C 3 photosynthesis was discovered before C 4 photosynthesis (M. Calvin). It is C 3 photosynthesis that is described above, under the heading “Dark phase”. Characteristic features of C 3 photosynthesis: 1) the carbon dioxide acceptor is RiBP, 2) the carboxylation reaction of RiBP is catalyzed by RiBP carboxylase, 3) as a result of carboxylation of RiBP, a six-carbon compound is formed, which decomposes into two PGAs. FGK is restored to triose phosphates(TF). Some of the TF is used for the regeneration of RiBP, and some is converted into glucose.

1 - chloroplast; 2 - peroxisome; 3 - mitochondria.
This is a light-dependent absorption of oxygen and release of carbon dioxide. At the beginning of the last century, it was established that oxygen suppresses photosynthesis. As it turned out, for RiBP carboxylase the substrate can be not only carbon dioxide, but also oxygen:
O 2 + RiBP → phosphoglycolate (2C) + PGA (3C).
The enzyme is called RiBP oxygenase. Oxygen is a competitive inhibitor of carbon dioxide fixation. The phosphate group is split off and the phosphoglycolate becomes glycolate, which the plant must utilize. It enters peroxisomes, where it is oxidized to glycine. Glycine enters the mitochondria, where it is oxidized to serine, with the loss of already fixed carbon in the form of CO 2. As a result, two glycolate molecules (2C + 2C) are converted into one PGA (3C) and CO 2. Photorespiration leads to a decrease in the yield of C3 plants by 30-40% ( With 3 plants- plants characterized by C 3 photosynthesis).
C 4 photosynthesis is photosynthesis in which the first product is four-carbon (C 4) compounds. In 1965, it was found that in some plants (sugar cane, corn, sorghum, millet) the first products of photosynthesis are four-carbon acids. These plants were called With 4 plants. In 1966, Australian scientists Hatch and Slack showed that C4 plants have virtually no photorespiration and absorb carbon dioxide much more efficiently. The pathway of carbon transformations in C 4 plants began to be called by Hatch-Slack.
C 4 plants are characterized by a special anatomical structure of the leaf. All vascular bundles are surrounded by a double layer of cells: the outer layer is mesophyll cells, the inner layer is sheath cells. Carbon dioxide is fixed in the cytoplasm of mesophyll cells, the acceptor is phosphoenolpyruvate(PEP, 3C), as a result of carboxylation of PEP, oxaloacetate (4C) is formed. The process is catalyzed PEP carboxylase. Unlike RiBP carboxylase, PEP carboxylase has a greater affinity for CO 2 and, most importantly, does not interact with O 2 . Mesophyll chloroplasts have many grains where light phase reactions actively occur. Dark phase reactions occur in the chloroplasts of the sheath cells.
Oxaloacetate (4C) is converted to malate, which is transported through plasmodesmata into the sheath cells. Here it is decarboxylated and dehydrogenated to form pyruvate, CO 2 and NADPH 2 .
Pyruvate returns to the mesophyll cells and is regenerated using the energy of ATP in PEP. CO 2 is again fixed by RiBP carboxylase to form PGA. PEP regeneration requires ATP energy, so it requires almost twice as much energy as C 3 photosynthesis.
The meaning of photosynthesis
Thanks to photosynthesis, billions of tons of carbon dioxide are absorbed from the atmosphere every year and billions of tons of oxygen are released; photosynthesis is the main source of the formation of organic substances. Oxygen forms the ozone layer, which protects living organisms from short-wave ultraviolet radiation.
During photosynthesis, a green leaf uses only about 1% of the solar energy falling on it; productivity is about 1 g of organic matter per 1 m2 of surface per hour.
Chemosynthesis
The synthesis of organic compounds from carbon dioxide and water, carried out not due to the energy of light, but due to the energy of oxidation of inorganic substances, is called chemosynthesis. Chemosynthetic organisms include some types of bacteria.
Nitrifying bacteria ammonia is oxidized to nitrous and then to nitric acid (NH 3 → HNO 2 → HNO 3).
Iron bacteria convert ferrous iron into oxide iron (Fe 2+ → Fe 3+).
Sulfur bacteria oxidize hydrogen sulfide to sulfur or sulfuric acid (H 2 S + ½O 2 → S + H 2 O, H 2 S + 2O 2 → H 2 SO 4).
As a result of oxidation reactions of inorganic substances, energy is released, which is stored by bacteria in the form of high-energy ATP bonds. ATP is used for the synthesis of organic substances, which proceeds similarly to the reactions of the dark phase of photosynthesis.
Chemosynthetic bacteria contribute to the accumulation of minerals in the soil, improve soil fertility, promote wastewater treatment, etc.
Go to lectures No. 11 “The concept of metabolism. Biosynthesis of proteins"
Go to lectures No. 13 “Methods of division of eukaryotic cells: mitosis, meiosis, amitosis”