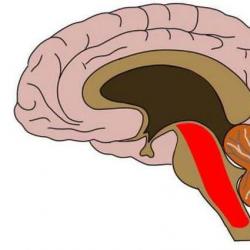
What is the reticular formation. Functions of the reticular formation of the brainstem The role of the reticular formation of the brainstem
Reticular formation of the brainstem is a complex of neurons that have extensive connections with different nerve centers, with each other and with the cerebral cortex. It runs rostrally to the thalamus. Let's consider its features further.
Functions of the reticular formation
The tasks of the complex include processing sensory information. In addition, the reticular formation provides an activating effect on the cortex, controlling the activity of the spinal cord. Due to this, the tone of skeletal muscles and the functioning of the human autonomic and reproductive systems are regulated.
Mechanism of action
It was first identified by R. Granite. The scientist found that he could influence the activity of γ-motoneurons. As a result, γ-efferents (their axons) provoke contraction of muscle spindles and, accordingly, an increase in afferent impulses of muscle receptors. Signals entering the spinal cord provoke excitation of α-motoneurons. This determines muscle tone. It was found that neurons of the pons and medulla oblongata formation are involved in the implementation of this function. Their behavior is diametrically opposed. The latter provoke activation of α-motoneurons in the flexor muscles and, accordingly, inhibit them in the extensors. Pontine neurons do the opposite. Reticular formation connected to the cerebellum and cortex, from which information comes. This allows us to conclude that it acts as a collector of nonspecific sensory flow, which may be involved in the regulation of muscle activity. However, at present, the need for a formation that duplicates the tasks of neurons in the red and vestibular nuclei has not yet been clarified.

Structure
Reticular formation formed by scattered cells. Some of them are considered vital formations. In particular, the following centers can be distinguished:
- Respiratory and vasomotor. They are located in the medulla oblongata.
- Gaze coordination. It is located in the midbrain.
- Hunger, satiety and thermoregulation. They are located in the diencephalon.
The key tract is the reticulospinal one. It passes to neurons in the motor nuclei of the anterior spinal horns and cranial nerves along the trunk and to the intercalary elements of the nervous autonomic system. Thalamocortical fibers extend from them. They provide activation of the cortex, which is necessary for the perception of specific stimuli. These thalamo-cortical fibers terminate in all cortical layers.

Scientific observations
During the research it was revealed that ticular formation has an activating effect on the cortex. This neural complex acts as a kind of “energy center”. Without it, the nerve cells of the cortex, its various sections, as well as the entire brain as a whole, will not be able to perform all of their diverse complex tasks. A complex of neurons is directly involved in the process of regulating sleep and wakefulness. The experimental results made it possible to explain some of the surgeons' observations. Thus, during brain surgery, incisions can be made in the cerebral cortex and part of the tissue can be removed. In this case, the patient will not lose consciousness. However, if the scalpel is touched, the person will fall into deep sleep.
Work specifics
Today, the specific nerve channels through which information is transmitted from the sensory organs to the brain are quite well studied. This is how the cortex learns about the nature of the stimulus acting on the body. In accordance with this, it sends different impulses to systems and organs. Studies have shown that all fibers directed from the periphery to the cortex have branches. They end on the surface of the cells of the formation. External irritation of any nature has a stimulating effect on her. At this moment, a kind of “energy charging” occurs. Acting as a brain center, the formation determines the degree of efficiency of the cortex. By activating all departments, it provides accurate synthesis and analysis of the variety of information that enters the cortex from the outside world.

Reaction to body substances
The reticular formation is sensitive not only to nerve signals, but also to compounds dissolved in the blood. In particular, we are talking about sugar, hormones, carbon dioxide, oxygen. Of particular importance among these substances is adrenaline. With emotional overstrain - with anger, fear, a state of passion, rage - there is a prolonged excitation of the formation. It is supported by adrenaline, which is intensely released into the blood. The activity of the complex is largely determined by other chemical compounds. First of all, these are carbon dioxide and oxygen. For example, if a person has difficulty breathing during sleep, then CO 2 begins to accumulate in the blood. Carbon dioxide activates the reticular formation, as a result of which a person wakes up.

Conclusion
Clinical studies and experimental data obtained in physiological laboratories have shown that the reticular formation is directly related to the occurrence of emotions. The results of studying its structure and the tasks it implements are widely used in psycho- and neuropharmacology. It was found that lethargy, apathy, drowsiness or irritability, insomnia can be caused by a disorder in the functioning of the reticular formation. This neuronal complex also plays a certain role in the process of occurrence of many pathologies of the central nervous system.
RETICULAR FORMATION [formatio reticularis(PNA, JNA, BNA); syn.: reticular substance, reticular formation] - a set of structures located in the central parts of the spinal cord and brain stem. The Reticular Formation is characterized by the presence of a large number of nerve fibers passing in a variety of directions, and therefore this formation under a microscope resembles a mesh. This served as the basis for Deiters (O. F. C. Deiters) to call it a reticular formation.
Morphology
At the end of the 19th - beginning of the 20th century. the outer section of the Reticular Formation of the medulla oblongata, consisting mainly of gray matter (formatio reticularis grisea), the inner section, consisting mainly of white matter (formatio reticularis alba), the lateral section (substantia reticularis lateralis), as well as individual nuclei of the Reticular Formation were described. The first systematic description of the nuclei of the Reticular Formation of the brain stem was given in 1909 by H. Jacobsohn.
The atlas of Meessen and Olszewski (Meessen, J. Olszewski, 1949) describes 32 nuclei of the rhomboid brain of the rabbit. In his 1954 work, Olshevsky describes 22 nuclei of R. f. medulla oblongata, pons and midbrain in humans.
The study of the structure of individual nuclei of the Reticular Formation and their constituent neurons, as well as the connections of the Reticular Formation, served as the basis for the division of R. f. into zones, parts or columns.
Comparative anatomical, morphological and ontogenetic study of R. f. allowed V.V. Amunts (1966, 1976, 1980) to group the nuclei of R. f. into 3 sections: external, internal and middle - and highlight the transition zones between reticular and specific, as well as between various reticular structures.
Characteristics of the neural structure of R. f. was made by Scheibel and Scheibel (A. B. Scheibel, M. E. Scheibel, 1962), G. P. Zhukova (1977), T. A. Leontovich (1978), H. Mannen (1966, 1975), Valverde (F Valverde, 1961), N. S. Kositsyn (1976), etc.
Nerve connections of R. f. were studied mainly by degeneration methods. Fibers R. f. They are divided into radial fibers directed ventrally, concentric fibers crossing in the midline area, and longitudinal fibers grouped in bundles. These bundles constitute both afferent and efferent pathways. Data on connections of R.f. summarized in the works of Brodal (1957), Rossi and Zanchetti (G. F. Rossi, A. Zanchetti, 1957).
Physiology
In the area of the brain stem there are anatomical formations, the stimulation of which has a generalized tonic effect on the anterior parts of the brain (see). This group of anatomical formations is called the ascending activating system of the reticular formation (Fig. 1). It plays an important role in maintaining a wakeful state, as well as in the mechanisms of formation of holistic, and in particular conditioned reflex reactions of the body. Along with the ascending activating system, there are also descending reticulospinal systems that have a controlling effect on the reflex activity of the spinal cord. The activity of both ascending and descending systems is supported by a continuous influx of afferent impulses entering the R. f. along collateral fibers from sensory pathways. An important role in maintaining the activity of reticular mechanisms is played by humoral irritants, in relation to the Crimea R. f. has high sensitivity, which ensures its participation in the regulation of a number of autonomic functions. Along with this, R. f. is the site of selective action of many pharmacological agents, which is widely used in the treatment of a number of diseases of c. n. pp., and also determines a new approach to the study of such important problems of medicine as, for example, the problem of pain and anesthesia. In the field of R. f. there is an extensive spatial coincidence and interaction of impulses coming from various peripheral receptor formations with excitations coming from the cerebellum and the cerebral cortex. Due to the large number of corticoreticular connections, the cerebral cortex has a controlling influence on the activity of the reticular mechanisms, regulating the level of their activity.
Reticulospinal relationships. The influence of the brain stem on the motor activity of the spinal cord was first demonstrated by I. M. Sechenov in 1862 (see Sechenov inhibition). They showed that when the brain stem is irritated, both inhibition and facilitation of reflex reactions can be observed. However, the mechanism and structures mediating these influences remained unclear. In 1944, X. Megun, using direct electrical stimulation of the brain stem, showed that irritation of certain areas of the bulbar R. f. leads to a complete stop of movements caused both reflexively and by irritation of the motor areas of the cerebral cortex. This inhibition was general and applied to all muscle groups, regardless of their topographic relationships and physiological function. X. Megun suggested that the controlling influence of R. f. carried out at the level of the spinal cord, and not at the level of the cerebral cortex. This assumption was confirmed by him in experiments on decerebrate animals.
There is no consensus regarding the mechanism of reticulospinal influences. While some researchers believe that R. f. may have an effect directly on the motor neurons of the spinal cord, others suggest that these influences are transmitted to the motor neurons through some intermediate neurons, the role of which can be played by interneurons involved in the closure of segmental spinal reflex arcs.
Reticulocortical relationships and the problem of the ascending activating influence of reticular mechanisms on the cerebral cortex. It is a known fact that the main symptoms of damage to certain areas of the brain stem are a general decrease in the patient’s activity, adynamia, and drowsiness. Experiments have shown that such phenomena can develop when the subcortical-stem parts of the brain are destroyed in an animal. These data served as a basis for a number of researchers to believe that in the area of the brain stem there are centers in charge of the general activity of the body, centers of sleep and wakefulness (see Centers of the nervous system). This assumption was justified by the fact that direct irritation of certain parts of the brain stem could induce sleep in an experimental animal or bring it out of this state. However, real progress in this problem became possible only after electrophysiological methods, in particular electroencephalography, began to be increasingly used for brain research (see). Research by V.V. Pravdich-Neminsky (1925) showed that external stimuli cause characteristic EEG changes, consisting in the replacement of slow high-amplitude and low-frequency oscillations characteristic of a resting state, with fast low-amplitude and high-frequency oscillations. Such EEG changes are observed in a person or animal during the transition from sleep to wakefulness. In this regard, this type of reaction is called the “EEG activation reaction”, or “awakening reaction”.
The development of experimental research methods has made it possible to develop methods for subtle stimulation and destruction of individual subcortical structures using electrodes inserted with great precision into the points required by the experimenter (see Stereotactic method). This allowed J. Moruzzi and X. Megun in 1949 to approach the question of which brain structures are responsible for the occurrence of the “awakening reaction.” To solve this problem, J. Moruzzi and X. Megun conducted a series of experiments, during which they established that when certain points of the brain stem are irritated in animals, there is a change from slow synchronous high-voltage oscillations characteristic of sleep to low-amplitude high-frequency activity. These EEG changes were diffuse, i.e., they were observed throughout the cerebral cortex, but were better expressed in the hemisphere of the same name in relation to the site of irritation. Changes in the electrical activity of the cortex of the greater hemungaria were accompanied by external signs of awakening.
Further research has shown that similar phenomena can be observed when various parts of the R. f. are irritated. brain stem - starting from the medulla oblongata and ending with the diencephalon. In the region of the medulla oblongata (see), the excitable zone coincides with those areas of the R. f., which, according to X. Megun and R. Rhines, also have a descending effect on the activity of the spinal cord. At the level of the pons (see Bridge of the brain) and the midbrain (see), this zone is located in the tegmental region, and at the level of the diencephalon (see) it captures the subthalamic nucleus and the posterior hypothalamus (see), reaching the medial thalamic nuclei. These structures of the brain stem constitute the ascending activating reticular system, the anatomical substrate of which is a series of ascending short-axon reticular tracts. X. Megun and J. Moruzzi came to the conclusion that the EEG changes they observed were not the result of antidromic conduction of impulses to the cerebral cortex along the known corticofugal pathways. These changes could not be explained by the conduction of excitation to the cortex along the known classical sensitive (lemniscal) pathways, since even after cutting these pathways, irritation of the medulla oblongata structures continued to cause distinct EEG changes.
The ideas of G. Moruzzi and X. Megun about the activating system of R. f. brainstem were further developed and confirmed in work carried out in laboratories in many countries around the world. The main conclusions of J. Moruzzi and X. Megun were confirmed and the topography of the structures attributed to the ascending activating reticular system of the brain stem was clarified.
Electrophysiological experiments conducted using microelectrode technology have shown that impulses from various peripheral sources and the cerebral cortex can converge to the same neuron of the Reticular Formation. The interaction between these impulses apparently determines the variety of observed effects from external stimuli.
The works of Dell (R. Dell) and his school show that an important role in maintaining the activity of R. f. belongs to humoral factors, in particular adrenaline. It was found that the activating effect of adrenaline on the cerebral cortex is carried out through R. f. midbrain and pons (Fig. 2). After transection of the brain stem anterior to the midbrain, administration of adrenaline no longer caused the “wake-up reaction.” Thus, the rostral parts of the brain stem have increased sensitivity to adrenaline. Moreover, studies by Vogt (M. Vogt, 4954) and P.K. Anokhin (1956) in the rostral parts of the brain stem revealed the presence of a large number of elements containing adrenaline and norepinephrine. IP Anokhina (1956) found that aminazine, which has the ability to block alpha-adrenergic receptors, prevents the development of EEG activation during painful stimulation. This gives reason to assume that the activating influence of R. f. on the cerebral cortex during painful stimulation is carried out due to the involvement of α-adrenergic receptors in the rostral parts of the brain stem in the activity.
What are the pathways through which reticular mechanisms influence the cerebral cortex? X. Megun and G. Moruzzi (1949) suggested that one of the ways through which R. f. has an effect on the cerebral cortex, is the group of medial nuclei of the thalamus (see), making up the so-called. nonspecific projection thalamocortical system. This system includes (in cats) nucleus reticularis, n. ventralis anterior, n. centralis medialis, n. lateralis, n. medialis, n. centrum medianum, n. parafascicularis and other nuclei of the intralaminar complex (intralamellar nuclei of the thalamus, T.). When nonspecific nuclei of the thalamus are irritated, generalized changes in the electrical activity of the cerebral cortex are observed, regardless of which of the nuclei is exposed to irritation; the nuclei of this system have a large number of connections among themselves, due to which, when excited, this system reacts as a single unit.
Studying the functional relationships between the ascending activating system of R. f. a large number of works are devoted to the brain stem and the nonspecific projection (diffuse) thalamic system. Many researchers simply combine these systems into one, given their close morphological unity. However, J. Moruzzi (1958) believes that there are no sufficient grounds for such a union and it is more appropriate to attribute the nonspecific thalamic system to the “spheres of influence” of R. f., especially since there are experimental data indicating reciprocal relationships between these formations. A detailed study of the paths connecting R. f. brain stem with nonspecific nuclei of the thalamus, carried out by Papez (J. W. Papez, 1956). According to his data, there are three such pathways: reticular-thalamic, passing from the R. f. medulla oblongata to the centromedial nucleus of the thalamus, as well as to the posteromedial and parafascicular nuclei. Sensitive pathways (lemniscal system) run along the lateral side of this path, from which collaterals extend to the tegmental nuclei. The tegmental-thalamic pathway begins from the tegmental nuclei, ending in the central median nucleus (centrum medianum) and the cells adjacent to it. The most lateral in location is the tectothalamic tract, ending in the borderline nucleus of the thalamus. Thanks to these pathways, the nonspecific projection thalamic system becomes a link between the ascending activating reticular system of the brain stem and the cerebral cortex.
Recognition of the existence of two afferent systems (specific and nonspecific), having different forms, methods and spheres of influence, led to the need to study the characteristics of the endings of the fibers of both of these systems in the cerebral cortex. It has been shown that the nerve fibers of these systems have terminals in the cortex that differ in shape and distribution in different cortical layers. Specific afferent fibers end predominantly in the 4th layer of the cortex, nonspecific fibers - in all layers of the cortex. Specific fibers end mainly on the cell body, nonspecific fibers - on its dendrites. Axodendritic endings of nonspecific fibers can create changes in the excitability of cortical neurons, facilitating or hindering synaptic transmission (see Synapse). These influences are diffuse and changeable. Axosomatic endings of specific fibers provide rapid and local responses. The interaction of both of these systems, according to Chang (H. T. Chang, 1952), determines the final reaction of cortical neurons.
It should be noted that if in the 50s and 60s. 20th century the view prevailed, according to R. f. was considered as a diffusely organized bark, exerting a “nonspecific” ascending and descending influence, then in the 70s. this opinion began to be revised.
One of the first researchers to point out that the activating influences of R. f. always have a certain biological “sign”, was P.K. Anokhin (1958). The reason for the radical revision of views was the progress of both physiological and morphological research methods. The latter, first of all, should include the improvement of the histofluorescence analysis technique, which made it possible to identify neurons in the brain stem containing monoamines (norepinephrine, serotonin and dopamine) and show the nature of the branching of the fibers of these neurons. It was noted that the norepinephrine and serotonin systems contain typical reticular neurons. These data forced us to reconsider the morphological dogma, according to which all pathways to the cerebral cortex are switched in the thalamus. It was found that mesencephalic serotonergic and noradrenergic neurons ascend directly from the nuclei of the R. f. into the cerebral cortex. In this regard, the question arose about the neurochemical basis of the cortical “awakening reaction”.
A significant contribution to the physiology of the Reticular Formation was made by the use of microelectrode research methods (see). It has been shown that one of the important mechanisms activating reticulothalamic and reticulocortical influences is the suppression of inhibitory interneurons, i.e. “inhibition of inhibition.”
New data were obtained in the process of studying reticulomotor coordination, i.e., in identifying local groups of cells specifically associated with the control of certain forms of motor activity, in particular in identifying neurons that regulate eye movements, neurons that control the mechanisms of posture and locomotion, and etc.
Discovery in a number of structures of R. f. high concentrations of the so-called. opiate receptors, indicating a connection with the organization of pain sensitivity. These structures include the nuclei of the median raphe, as well as the central gray matter around the Sylvian aqueduct, etc.
Detection of functional specialization of various departments of R. f. led to a reassessment of the attitude towards R. f. as a “non-specific system”. A special international symposium was organized and held in the USA, the materials of which were published in the monograph “Revision of views on the reticular formation: the specific function of a nonspecific system” (1980).
However, it should be noted that studies summarizing information about R. f. do not cancel the view of R. f. as a system that works in functional unity with the analytical systems and exerts tonic influences on the underlying and overlying sections of the c. n. With.
It is impossible not to take into account that long before the appearance of the first studies on R. f., I. M. Sechenov showed that in the area of the brain stem there are structures that control the activity of the spinal cord. I.P. Pavlov also attached great importance to subcortical formations in maintaining the activity of the cerebral cortex. He spoke about the “blind power” of the subcortex, about the subcortex as “a source of energy for the cortex.” Establishment of the important role of reticular mechanisms in the activity of c. n. With. is a concretization of the theoretical ideas of these brilliant Russian scientists.
Pathology of the reticular formation
Dysfunction of R. f. develops as a result of damage to its nuclei, localized, ch. arr., in the region of the medulla oblongata, pons and midbrain, as well as afferent and efferent connections at various levels.
The pathology of the integrative functions of R. f., as shown by A. M. Wayne (1974), Eliasson (S. G. Eliasson, 1978), can manifest itself in the form of movement disorders (see), disturbances of consciousness (see), sleep (see. ), autonomic dysfunction.
Movement disorders are caused by a violation of the phasic and tonic control of striated muscles, which is normally carried out through the interaction of the activating and inhibitory influences of R. f., transmitted to the alpha and gamma motor neurons of the spinal cord (see) through the reticulospinal and vestibulospinal tracts.
Pathology R. f. brain stem, as well as its afferent and efferent connections, can be accompanied by both an increase in muscle tone and tendon reflexes, and their decrease. Muscular hypertension and increased tendon reflexes arise due to the predominance of the activating influences of the reticular formation on the a- and γ-motoneurons of the spinal cord, which is observed when the giant cell reticular nucleus is damaged at the level of the medulla oblongata or pons, its afferent connections with the cerebral cortex and the caudate nucleus, as well as with pathology of the efferent pathways to the motor neurons of the spinal cord.
Diffuse defeat of R. f. at the level of the brain stem can lead to a sharp decrease in muscle tone and tendon reflexes due to the lack of activating influences on the a- and 7-motoneurons of the spinal cord.
Movement disorders in the pathology of R. f. concern not only the striated muscles of the trunk and limbs, but also the muscles innervated by the cranial (cranial, T.) nerves.
Another wedge, a syndrome observed in the pathology of R. f., is a disorder of consciousness up to the appearance of coma. Coma (see) is characterized by complete loss of consciousness, lack of response to external stimuli, and a slow synchronized EEG rhythm. The basis of coma is the blockade of the ascending activating RF, which is responsible for the processes of wakefulness and activation of attention to various sensory stimuli. Functional or structural disturbances of the ascending activating system at any level, including the oral brainstem, septum pellucidum, hypothalamus, thalamus, and thalamocortical connections, can lead to disorders of consciousness. Comatose states most often develop with pathology of the brain stem and midbrain or with processes leading to their dislocation. But another mechanism of coma is also possible, in which it is based on a pathology of the cerebral cortex and a violation of the descending influences of the cortex on the R. f., as a result of which the functional state of the R. f. changes for the second time.
To defeat R. f. in the area of the tegmentum of the pons and midbrain, pseudocoma syndrome, or akinetic mutism, is characteristic. Akinetic mutism syndrome is characterized by a loss of the ability to adequately respond to external stimuli with intact consciousness or a mild impairment thereof. At the same time, the patient’s speech (see), active movements are impaired, he does not remember events occurring in a given period of time. Pupillary reactions, tendon and periosteal reflexes do not change. Strong pain and sound stimulation cause a motor response. The syndrome is based on a violation of the ascending activating system and its connections with the limbic structures of the brain (see Limbic system), which leads to a lack of motivation to action, difficulty integrating motor functions, and memory impairment (see).
A frequent symptom of R. f. and its connection is sleep disorder. Sleep pathology (see) is caused either by a violation of the functional reciprocal relationships between the ascending activating system and the hypnogenic synchronizing zones responsible for generating sleep, or by dysfunction of the hypnogenic zones themselves, located hl. arr. within the limbic-reticular complex.
Sleep disorders can be in the form of increased sleepiness (hypersomnia) and various night sleep disorders (insomnia). Hypersomnias can be caused by hypofunction of the ascending activating system or hyperfunction of one of the systems of sleep regulation mechanisms. Hypersomnic states observed with organic damage to the mesencephalic-diencephalic region of the brain are the result of a disruption in the functioning of the activating system of R. f. Insomnia, characterized by insomnia, difficulty falling asleep, frequent waking up, shortening the duration of night sleep, can be caused by relatively increased functional activity of the ascending activating system, as well as impaired functioning of individual somnogenic areas of the brain responsible for the generation of REM and slow-wave sleep. Such hypnogenic zones are located in the caudal part of the brain stem (the so-called synchronizing system of G. Moruzzi), in the nuclei of the median raphe, and the hypothalamus. With their pathology, disorganization of individual phases of sleep is observed. Disruption of the cyclic organization of sleep without gross changes in individual phases is characteristic of the pathology of the integrating apparatuses that regulate the activation of synchronizing and desynchronizing sleep mechanisms. These include individual structures of the limbic-reticular complex (hypothalamus, thalamus optic, basal ganglia of the telencephalon), as well as the activating thalamocortical system.
In case of dysfunction of R. f. vegetative-vascular dystonia syndrome may be observed in the brain stem. According to A. M. Vein et al. (1981), vegetative-vascular disorders are observed in the majority of patients with pathology of the brain stem. Autonomic disorders are represented by cardiovascular, vasomotor and respiratory disorders, which may have a sympathetic-adrenal or parasympathetic orientation (see Neurocirculatory dystonia). The basis of vegetative disorders that occur when R. is affected. brain stem, there is not only dysfunction of specific autonomic centers (vasomotor, respiratory), but also a violation of the holistic integrative function necessary to ensure appropriate adaptive behavior. Therefore, respiratory, cardiovascular and vasomotor disorders observed in the pathology of R. f. brain stem, are accompanied by changes in muscle tone, motility and secretion of internal organs, endocrine disorders, mood changes, and memory loss. There is a certain dependence of the wedge, manifestations of vegetative-vascular dystonia on the affected area of R. f. brain stem. When the upper parts of the brainstem are disturbed, autonomic disorders are of a sympathetic nature and may be accompanied by mild neuroendocrine disorders. In patients with damage to the caudal parts of the trunk, the parasympathetic direction of the tone of the autonomic nervous system is revealed, and vestibular disorders are often observed. This is due to the presence of connections of R. f. with the nuclei of the vagus nerve and vestibular nuclei.
Study of the physiology and pathophysiology of R. f. allowed us to significantly deepen our understanding of the mechanisms of development of many diseases of the nervous system. Pathology of the ascending activating system of R. f. underlies disturbances of consciousness and comatose states (in acute cerebrovascular accidents, traumatic brain injuries, tumors, encephalitis, metabolic disorders). Blockade of activating influences on the cerebral cortex can be caused either directly by the lesion itself, localized in the brain stem, midbrain or hypothalamus, or by edema, leading to compression, dislocation and secondary metabolic disorders in this area.
In comatose states caused by metabolic disorders (eg, hypoglycemia) or drug intoxication (barbiturates, tranquilizers, adrenolytic drugs), direct suppression of neurons of the activating reticular system or blockade of adrenergic receptors of synapses is observed.
An increase in the functional activity of the waking system, manifested by sleep pathology in the form of insomnia, is characteristic of neuroses. In patients with neuroses (see), the syndrome of vegetative-vascular dystonia and emotional disorders characteristic of dysfunction of the limbic-reticular complex are also often observed.
Pathology R. f. plays a certain role in the development of parkinsonism syndrome (see). Among the typical morphological changes in this disease, the death of neurons of the R. f., which ensures a state of wakefulness, is often detected. Increased drowsiness and akinesia in parkinsonism depend not only on the primary lesion of the activating system of the R. f., but also on its blockade due to the functional enhancement of the inhibitory influences of the caudate nucleus at the level of the reticulocortical connections.
Pathology of descending influences of R. f. plays a role in the formation of central paralysis and paresis, extrapyramidal rigidity, myoclonus.
Clarification of the function of the R.f. and clarification of its role in the development of patol. disorders became possible on the basis of extensive experimental and clinical research. To study the function of R. f. the method of implantation of electrodes with determination of the activity of cell populations, electroencephalographic analysis, morphological studies using electron microscopy (see), methods of histochemistry (see) and biochemistry (see), including the study of the neurochemistry of mediators (see), are used. In wedges, practice, polygraphic research methods are widely used, including simultaneously electroencephalography (see), electrooculography (see), electromyography (see), electrocardiography (see), with the help of which it is possible to differentiate the level of damage to the nervous system, functional state of ascending and descending systems of R. f. and identify the features of their response to the use of various pharmacological agents.
Treatment
There is a wide arsenal of pharmacological drugs that affect the function of R. f. and its connections with other brain structures. Barbiturates have a selective effect on the activating reticular system, which blocks ascending impulses to the cerebral cortex. This mechanism underlies their narcotic and anticonvulsant effects. A direct inhibitory effect on the ascending activating system is exerted by bromine drugs, phenothiazine drugs (aminazine, etc.), certain tranquilizers (chlordiazepoxide, diazepam, oxazepam, nitrazepam), which is associated with their calming, anticonvulsant and mild hypnotic effect. The ascending reticular system is activated by adrenergic mediators (adrenaline, norepinephrine), their precursor L-DOPA, as well as indirect adrenergic agonists (caffeine, nialamide, imizin, amitriptyline, phenamine, meridil, sydnocarb, etc.). These drugs are used in complex therapy of patients in a comatose state, with increased drowsiness, depression, and asthenia. Cholinergic synapses of the reticular formation are blocked by central cholinomimetics (scopolamine, amizil, meta-lysil), which leads to a decrease in the parasympathetic effects of R. f. brain stem to internal organs. A decrease in the flow of sympathetic impulses to the periphery can be achieved by using sympatholytic drugs (reserpine, methyldopa), which disrupt the formation of catecholamines and activate the inhibitory structures of R. f. There are drugs that have a selective effect on the serotonergic structures of R.f. (L-tryptophan, dyseril). These drugs are used clinically to normalize sleep. The effect of inhibition of reticular cells of the caudal brain stem with drugs such as lioresal, midocalam, diazepam is used in the treatment of patients with increased muscle tone.
Correction of syndromic disorders of R. f. is part of the complex therapy of diseases of the nervous system, in which the leading place should be given to etiological and pathogenetic treatment.
Bibliography: Amunts V.V. Development of the reticular formation of the brainstem in the ontogenesis of the lower ape compared to humans, Arkh. anat., histol. and emb-riol., t. 71, v. 7, p. 25, 1976, bibliogr.; Anokhin P.K. The significance of the reticular formation for various forms of higher nervous activity, Physiol. magazine USSR, vol. 43, no. 11, p. 1072.1957; o N e, Key issues of the theory of a functional system, M., 1980; Brodal A. Reticular formation of the brain stem, trans. from English, M., 1960, bibliogr.; Bein A. M. Lectures on the neurology of nonspecific brain systems, M., 1974; Vein A. M. and S o l o v e v a A. D. Limbic-reticular complex and autonomic regulation, M., 1973, bibliogr.; Vein A. M., S o l o v e v a A. D. and Kolosova O. A. Vegetovascular dystonia, M., 1981, bibliogr.; D e-m and N. N., Kogan A. B. and M o i-seeva N. I. Neurophysiology and neurochemistry of sleep, L., 1978; Zhukova G. P. Neural structure and interneuron connections of the brain stem and spinal cord, M., 1977, bibliogr.; Kositsyn N. S. Microstructure of dendrites and axodendritic connections in the central nervous system, M., 1976; M e g u n G. The Waking Brain, trans. from English, M., 1961; Reticular formation of the brain, ed. G. G. Jasper et al., trans. from English, M., 1962; Rossi D. F. and Ts a n k e t-t and A. Reticular formation of the brain stem, trans. from English, M., 1960, bibliogr.; Svyadosch A. M. Neuroses, M., 1982; Structure and function of the reticular formation and its place in the system of analyzers, ed. S. A. Sarkisova, M., 1959; Handbook of clinical neurology, ed. by P. J. Vinken a. G. W. Bruyn, v. 1, Amsterdam a. o., 1975; Meessen H.u. Olszewski J. Cytoarchitektonischer Atlas des Rautenhirns des Kaninchens, Basel - N. Y., 1949; M o r u z i G. a. M a- g o u n H. W. Brain stem reticular formation and activation of E E G, Electroen-ceph. clin. Neurophysiol., v. 1, p. 455, 1949; Neurological pathophysiology, ed. by S. G. Eliasson a. o., N.Y., 1978; O 1 s-zewski J. The cytoarchitecture of the human reticular formation, in the book: Brain mechanisms and consciousness, ed. by E. D. Adrian a. o., p. 54, Oxford, 1954, bibliogr.; Purpura D. P., Me Murtry J. G. a. Maekawa K. Synaptic events in ventrolateral thalamic neurons during suppression of recruitfing responses by brain stem reticular stimulation, Brain Res., v. 1, p. 63, 1966; R a-mon y Cajal S. Histologie du sys-teme nerveux de l'homme et des vertebres, t. 1-2, Madrid, 1952-1955; The reticular formation revisited, ed. by J. A. Hobson a. M. A. B. Brazier, N. Y., 1980, bibliogr.
V. V. Amunts, V. G. Skrebitsky, V. N. Shelikhov; L. O. Badalyan (neur.).
Reticular formation The brain stem is considered one of the important integrative apparatuses of the brain.
The actual integrative functions of the reticular formation include:
- control over sleep and wakefulness states
- muscle (phasic and tonic) control
- processing of information signals from the environment and internal environment of the body, which arrive through different channels
A large number of afferent pathways from other brain structures converge in the reticular formation: from the cerebral cortex - collaterals of the corticospinal (pyramidal) tracts, from the cerebellum and other structures, as well as collateral fibers that approach through the brain stem, fibers of sensory systems (visual, auditory, etc.). All of them end with synapses on neurons of the reticular formation. Thus, thanks to this organization, the reticular formation is adapted to combine influences from various brain structures and is able to influence them, that is, to perform integrative functions in the activity of the central nervous system, largely determining the general level of its activity.
Properties of reticular neurons. Neurons of the reticular formation are capable of stable background impulse activity. Most of them constantly generate discharges with a frequency of 5-10 Hz. The reason for this constant background activity of reticular neurons is: firstly, the massive convergence of various afferent influences (from receptors of the skin, muscle, visceral, eyes, ears, etc.), as well as influences from the cerebellum, cerebral cortex, vestibular nuclei and others brain structures onto the same reticular neuron. In this case, excitement often arises in response to this. Secondly, the activity of the reticular neuron can be changed by humoral factors (adrenaline, acetylcholine, CO2 tension in the blood, hypoxia, etc.). These continuous impulses and chemicals contained in the blood support the depolarization of the membranes of the reticular neurons, their ability to impulse activity. In this regard, the reticular formation also has a constant tonic effect on other brain structures.
A characteristic feature of the reticular formation is also the high sensitivity of its neurons to various physiologically active substances. Due to this, the activity of reticular neurons can be relatively easily blocked by pharmacological drugs that bind to the cytoreceptors of the membranes of these neurons. Particularly active in this regard are barbituric acid compounds (barbiturates), chlorpromazine and other drugs that are widely used in medical practice.
The nature of nonspecific influences of the reticular formation. The reticular formation of the brain stem is involved in the regulation of the autonomic functions of the body. However, back in 1946, the American neurophysiologist N. W. Megoun and his colleagues discovered that the reticular formation is directly related to the regulation of somatic reflex activity. The reticular formation has been shown to have diffuse, nonspecific, descending and ascending influences on other brain structures.
Downward influence. When the reticular formation of the hindbrain is irritated (especially the giant cell nucleus of the medulla oblongata and the reticular nucleus of the pons, where the reticulospinal tract originates), inhibition of all spinal motor centers (flexion and extension) occurs. This inhibition is very deep and long lasting. This situation can naturally occur during deep sleep.
Along with diffuse inhibitory influences, when certain areas of the reticular formation are irritated, a diffuse influence is revealed that facilitates the activity of the spinal motor system.
The reticular formation plays an important role in regulating the activity of muscle spindles, changing the frequency of discharges supplied by gamma efferent fibers to the muscles. In this way, the reverse impulse in them is modulated.
Rising influence. Research by N. W. Megoun and G. Moruzzi (1949) showed that irritation of the reticular formation (hindbrain, midbrain and diencephalon) affects the activity of the higher parts of the brain, in particular the cerebral cortex, ensuring its transition to an active state. This position is confirmed by numerous experimental studies and clinical observations. Thus, if the animal is in a state of sleep, then direct stimulation of the reticular formation (especially the pons) through electrodes inserted into these structures causes a behavioral reaction of awakening the animal. In this case, a characteristic image appears on the EEG - a change in the alpha rhythm by the beta rhythm, i.e. the reaction of desynchronization or activation is recorded. This reaction is not limited to a certain area of the cerebral cortex, but covers large areas of it, i.e. is of a generalized nature. When the reticular formation is destroyed or its ascending connections with the cerebral cortex are switched off, the animal falls into a sleep-like state, does not react to light and olfactory stimuli, and does not actually come into contact with the outside world. That is, the telencephalon stops actively functioning.
Thus, the reticular formation of the brain stem performs the functions of the ascending activating system of the brain, which maintains the excitability of neurons in the cerebral cortex at a high level.
In addition to the reticular formation of the brainstem, the ascending activating system of the brain also includes nonspecific nuclei of the thalamus, posterior hypothalamus, limbic structures. Being an important integrative center, the reticular formation, in turn, is part of more global integration systems of the brain, which include hypothalamic-limbic and neocortical structures. It is in interaction with them that appropriate behavior is formed, aimed at adapting the body to changing conditions of the external and internal environment.
One of the main manifestations of damage to the reticular structures in humans is loss of consciousness. It occurs with cerebrovascular accidents, tumors and infectious processes in the brain stem. The duration of the state of fainting depends on the nature and severity of dysfunction of the reticular activating system and ranges from a few seconds to many months. Dysfunction of ascending reticular influences is also manifested by loss of vigor, constant pathological drowsiness or frequent attacks of falling asleep (paroxysmal hypersomia), restless night sleep. There are also disturbances (usually increased) muscle tone, various autonomic changes, emotional and mental disorders, etc.
Summary: the biological basis of attention is the orienting reflex.
I.P. Pavlov described the orienting reflex as an unconditioned reflex that serves as the basis of involuntary attention. The very processes of attention in its system are explained, first of all, due to the interaction of excitation and inhibition occurring in the cerebral cortex. When a person is attentive to something, this means that a center of excitation arises in his cerebral cortex. At the same time, all other parts of the brain are in a state of inhibition. Therefore, a person focused on one thing may not notice anything else at that moment. But these ideas about brain relationships are too abstract. To see this, it is worth comparing this approach with the approach of A.R. Luria.
Teachings of A.R. Luria. In the teachings of A.R. Luria on the cerebral localization of higher mental functions of a person, a structural-functional model of the brain is given, in which each higher mental function is performed through the joint work of three brain blocks (Luria A.R. Fundamentals of Neuropsychology. M., 1973). The first block (the block for regulating the level of general and selective brain activation) is formed by nonspecific structures of the reticular formation of the brainstem, structures of the midbrain, diencephalic sections of the brainstem, limbic system, mediobasal sections of the cortex of the frontal and temporal lobes of the brain. The second block (the block for receiving, processing and storing modality-specific information) is formed by the main analyzer systems (visual, auditory, skin-kinesthetic), the cortical zones of which are located in the posterior parts of the cerebral hemispheres. The third block (the block of programming, regulation and control over the course of mental function, ensuring the formation of motives for activity and control over the results of activity through a large number of bilateral connections with cortical and subcortical structures) is formed by the motor, premotor and prefrontal sections of the cerebral cortex. At the same time, the sequence of work of these structures is important: at the first stage there is an incentive to activity, the basis of which is, among other things, the activation of the reticular formation.
The role of the reticular formation. The ability to be alert, sometimes reacting to a very slight change in the environment, is ensured by networks of nerve pathways located in the cerebral hemispheres that connect the reticular formation (a set of brain structures that regulate the level of excitability) with different parts of the cerebral cortex. Nerve impulses traveling along this network arise along with signals from the sensory organs and excite the cortex, bringing it into a state of readiness to respond to future stimuli expected. Thus, the reticular formation with its ascending and descending fibers, together with the sense organs, determines the appearance of the orienting (or orienting-exploratory) reflex, being the primary physiological basis of attention.
Back in 1935, F. Bremer compared electroencephalograms with two types of transection of the brain stem: a) at the level of the cervical vertebrae (a drug called “encephale isole” - the lower parts of the brainstem) and b) at the level of the bridge (the drug “cerveau isole” - upper parts of the trunk). In the first case, recordings of bioelectrical activity did not differ from the EEG of normal animals, while in the second case, slow waves of large amplitude, characteristic of the sleep state, were constantly present in the EEG. In preparations called "cerveau isole", only visual and olfactory afferent stimuli reach the cortex, since the signals transmitted by other cranial nerves (in particular, the auditory and trigeminal) are cut off. From this F. Bremer concluded that when the central nervous system is deprived of most of the stimulation coming from the external world, sleep occurs; accordingly, maintaining a state of wakefulness is the result of the activating influence exerted by sensations. As D. Lindsley later showed, in these cases, signals caused by sensory stimuli continue to reach the cortex, but the electrical responses of the cortex to these signals become only short-term and do not cause lasting changes. This showed that for the emergence of persistent processes of excitation that characterize the state of wakefulness, a single influx of sensory impulses is not enough; the supporting influence of the activating reticular system is necessary.
These ideas about the processes of general activation were further developed in the works of G. Moruzzi and G. Magoun (Moruzzi G., Magoun H.W. Brain stem reticular formation and activation of the EEG // EEG and Clinical Neurophysiology. 1949, 1 - “Reticular formation of the brain stem and activation reaction in the EEG"). They conducted experiments based on electrical stimulation of the brain, which revealed the functions of a nonspecific brain system - the reticular formation of the brain stem, which, along with the limbic system, is classified as a “modulating” brain system. The main function of these systems is to regulate the functional states of the body. The researchers did not turn off, but irritated the ascending reticular formation with electrodes implanted into it; they showed that such irritation of the reticular formation leads to the awakening of the animal, and further intensification of these irritations leads to the appearance of pronounced effective reactions of the animal. It turned out that when it is irritated by an electric current, an activation reaction occurs, and when this structure is removed, a coma occurs. These structures are actually responsible for maintaining the state of wakefulness, and the degree of their activity itself partly depends on sensory influences. However, contrary to what Bremer assumed, the activating influence of sensory does not manifest itself in the form of direct activation of the cerebral cortex by specific signals; it affects primarily the reticular formation, the activity of which in turn regulates the functional state of the cortex, motor and autonomic centers. It was found that the cortical sleep of Bremer's "cerveau isole" preparations was caused not by the cutting of specific sensory pathways to the cortex, but by the elimination of the influences exerted on it by the reticular formation.
Also, in the experiments of D. Lindsley, it was revealed that irritation of the stem nuclei of the ascending activating reticular formation significantly lowers the sensitivity thresholds (in other words, aggravates sensitivity) of the animal and allows for subtle differentiations (for example, differentiation of the image of a cone from the image of a triangle), which were previously inaccessible to the animal .
Neuroanatomy of the reticular formation. Initially, it was believed that the nonspecific brain system, which performs the task of diffuse and generalized activation of the cerebral cortex, included only network-like formations of the brain stem. It is now accepted that the ascending nonspecific activating system occupies a place from the medulla oblongata to the thalamus.
The reticular (from the Latin word reticulum - mesh) formation consists of numerous groups of neurons that do not have clear boundaries. Such a cluster of nerve cells, in its principle of organization, resembles the nerve networks of coelenterates. Their long and highly branched processes form networks around the gray matter of the spinal cord and in the dorsal part of the brainstem. It was first described in the middle of the 19th century, and the name of this structure was given by O. Deiters. In the reticular formation of the brain stem there are over 100 nuclei, which from the spinal cord to the diencephalon are combined into three main groups. 1) The median group of nuclei is concentrated around the midline, mainly in the region of the suture of the bridge and the medulla oblongata (suture nuclei), which are formed by fibers of the sensory pathways coming from the spinal cord, the nuclei of the trigeminal nerve and forming the decussation along the midline. 2) The medial group of nuclei is located on the sides of the previous one: it includes the medial magnocellular nucleus, locus coeruleus, neurons of the central gray matter of the midbrain, etc. 3) The lateral group of nuclei is located lateral to the medial one and includes the lateral reticular nucleus, parabrachial nuclei, etc.
The neurons of the reticular formation have different sizes: in the median and medial nuclei there are large nerve cells that form long afferent and efferent pathways, and in the lateral nuclei there are medium and small neurons, which are mainly associative neurons.
Most neurons of the reticular formation use peptides (enkephalins, neurotensin, etc.) as a transmitter of nerve impulses, but monoamines are also widely represented. The raphe nuclei contain serotonergic neurons, and the locus coeruleus contains noradrenergic neurons.
The connections of the reticular formation are divided into afferent and efferent. Afferent fibers end on its neurons: from the spinal cord, following the branches of all sensory pathways, as well as along the spinoreticular tract, from the nuclei of the cranial nerves as part of the collaterals of the nuclear-cortical, auditory and visual pathways, from the cerebellum as part of the cerebellar-reticular pathway, from the nuclei of the thalamus, subthalamus and hypothalamus, striatum, structures of the limbic system, various parts of the cerebral cortex, including branches of the corticospinal and corticonuclear tracts. Neurons of the reticular formation have long thin efferent processes, divided into ascending and descending branches, which are sent to various parts of the brain and spinal cord: motor neurons of the anterior horns of the spinal cord and motor nuclei of the cranial nerves of the brain stem as part of the reticulonuclear and reticulocerebellar tracts, cerebellum, red nucleus, substantia nigra and nuclei of the roof plate of the spinal cord, reticular nuclei of the thalamus, nuclei of the hypothalamus, indirectly through the nuclei of the diencephalon to the striatum, limbic system and new cortex.
With the help of the reticular formation, the motor and autonomic nuclei of the brain stem are combined into functional centers that regulate many complex forms of behavior: circulatory, respiratory, coughing, swallowing, vomiting, etc. The reticular formation ensures: 1) Maintaining a state of wakefulness. By increasing or decreasing the flow of sensory information to the cerebral cortex and subcortical structures, the reticular formation plays the role of a regulator of the level of consciousness (sleep/wake cycle). By regulating the neurotransmitter exchange of neurons in the reticular formation or modulating the activity of their receptors with the help of certain medications, it is possible to activate the activity of the cerebral cortex or, conversely, to achieve sleep. For example, caffeine contained in coffee or tea stimulates the nerve cells of the reticular formation. On the contrary, among psychotropic drugs (from the Greek psyche - soul + tropos - direction) there are so-called neuroleptics, which, by blocking the reticular formation of the brain and reducing the speed of excitation, act in a calming manner (suppress delirium, hallucinations, feelings of fear, aggressiveness, psychomotor agitation ). 2) Control of reflex activity by stimulating or inhibiting motor neurons of the anterior horns of the gray matter of the spinal cord and the motor nuclei of the cranial nerves of the brain stem. 3) Combining a group of neurons from different parts of the brain and spinal cord, which makes it possible to perform complex reflex acts: swallowing, chewing, coughing, vomiting, etc. 4) Providing autonomic regulation through the coordination of efferent and afferent signals in the corresponding centers of the brain stem. Thus, the vasomotor and respiratory centers unite groups of neurons responsible for the regulation of breathing and blood circulation. 5) Participation in the emotional perception of sensitive signals by increasing or decreasing the flow of afferent impulses to the limbic system.
The selective nature of the course of mental processes, which is characteristic of attention, is ensured only by the awake state of the cortex with an optimal level of excitability. This wakeful level is achieved due to the work of the mechanisms of communication of the upper trunk with the cerebral cortex and, above all, with the work of the ascending activating reticular formation. It is this ascending activating reticular formation that brings to the cortex, preserving it in a state of wakefulness, impulses associated with the metabolic processes of the body, drives, and exteroceptors that bring information from the outside world. First, this flow goes to the upper parts of the trunk and nucleus of the visual thalamus, and then to the cerebral cortex.
Ensuring optimal tone and wakefulness of the cortex is carried out, however, not only by the ascending activating reticular formation. The apparatus of the descending system is also closely connected with it, the fibers of which begin in the cerebral cortex (primarily in the medial and mediobasal sections of the frontal and temporal lobes) and are directed both to the nuclei of the brainstem and to the motor nuclei of the spinal cord. The work of the descending reticular formation is very important in that with its help, those forms of excitation that initially arise in the cerebral cortex and are the product of higher forms of conscious human activity with its complex cognitive processes and complex programs of actions formed during life are brought to the nuclei of the brain stem.
The interaction of both components of the activating reticular system provides the most complex forms of self-regulation of active states of the brain, changing them under the influence of both elementary (biological) and complex (social in origin) forms of stimulation.
Phylogenetically very ancient neural structure and well-developed part of the reptile brain stem. At first it was a polysynaptic pathway with slow conduction, closely connected with the olfactory and limbic areas. The progressive dominance of vision and hearing over the sense of smell led to a shift of sensory and motor functions into the midbrain tegmentum. The direct spino-tegmental and tegno-spinal tracts bypass the reticular formation, which is primarily responsible for autonomic regulation. In mammals, the tegmentum, in turn, began to play a secondary role in the transmission of excitation along very fast-conducting fibers connecting the cerebral cortex with peripheral motor and sensory neurons.
In the human brain, the reticular formation retains its connection with the limbic system and continues to play an important role in autonomic and reflex regulation.
Term reticular formation attributed only to the polysynaptic neural network of the brainstem, although the network extends anteriorly to the thalamus and hypothalamus and posteriorly to the propriospinal tract of the spinal cord.
General structure shown in the figure below. The median reticular formation is formed by a number of raphe nuclei (Greek -nuclei raphe). Most of the serotonergic pathways of the axial nervous system originate from the raphe nuclei.
Reticular formation (RF).(A) Departments. (B) Groups of aminergic and cholinergic cells.
The paramedian reticular formation is located nearby. This section consists entirely of magnocellular neurons; In the lower part of the pons and the upper part of the medulla oblongata (up to the level of fusion of the reticular formation with the central reticular nucleus of the medulla oblongata), giant cell neurons can also be found.
The most anterior section is considered lateral parvocellular reticular formation. The long dendrites of small cell neurons form branches at regular intervals. The dendrites have a predominantly transverse direction, and through the spaces between them there are long pathways to the thalamus. The lateral section is formed mainly by afferent neurons. They are approached by fibers from all sensitive pathways, including the sense organs.
Olfactory fibers pass through the medial forebrain bundle, located next to the hypothalamus.
The visual pathways pass through the superior colliculus.
The auditory fibers originate from the superior olivary nucleus.
Vestibular fibers originate from the medial vestibular nucleus.
Somatic sensory fibers pass through the spino-reticular tracts from the spinal and propria (principal or main pontine) nuclei of the trigeminal nerve.
Most of the axons of small cell neurons extensively branch between the dendrites of neurons of the paramedian reticular formation. However, some of them form synapses with the nuclei of cranial nerves and participate in the creation of movement programs.
Paramedian reticular formation- predominantly efferent system. The axons are relatively long, some ascend to form synapses with the reticular formation of the brainstem or the thalamus. From others, both ascending and descending branches depart, forming a polysynaptic network. Fibers from the premotor cortex, which give rise to the reticulospinal tracts of the pons and medulla oblongata, approach the magnocellular neurons.

A) Aminergic neurons of the brainstem. Scattered throughout the reticular formation are groups of aminergic (or monoaminergic) neurons - neurons whose mediators are formed from aromatic amino acids and have a number of effects on the cell. One group produces the neurotransmitter serotononin, three others produce catecholamines (dopamine, norepinephrine and adrenaline), and one group produces histamine.
 Serotonergic pathways from the midbrain stem (raphe).
Serotonergic pathways from the midbrain stem (raphe).
Serotonergic neurons- the most common neurons in any part of the central nervous system (CNS). These include neurons of the midbrain, the fibers of which rise to the cerebral hemispheres; pontine neurons branching in the brainstem and cerebellum; cells of the medulla oblongata descending into the spinal cord.
All parts of the gray matter of the central nervous system are penetrated by serotonin-secreting axonal branches. Increasing serotonergic activity is used in clinical practice to treat such a common disease as major depressive disorder.

Midbrain dopaminergic neurons presented in two groups. At the junction of the tire with the legs there is a black substance. Medial to it are the ventral tegmental nuclei, from which mesocortical fibers extend to the frontal lobe and mesolimbic fibers going directly to the nucleus accumbens.
Noradrenergic (norepinephrine) neurons slightly less numerous than serotonergic ones. About 90% of neuron cell bodies are concentrated in the locus ceruleus in the floor of the fourth ventricle at the upper end of the pons. Paths in all directions begin from the blue spot, as shown in the figure below.
 Noradrenergic pathways from the pons and medulla oblongata.
Noradrenergic pathways from the pons and medulla oblongata.
Adrenaline-secreting (epinephrine-secreting) neurons relatively few in number and located predominantly in the rostral/caudal regions of the medulla oblongata. One part of the fibers ascends to the hypothalamus, the other goes down, forming synapses with preganglionic sympathetic neurons of the spinal cord.
In the cerebral hemispheres, the ionic and electrical activity of aminergic neurons varies significantly. First, there is more than one type of postsynaptic receptor for each amine. Secondly, some aminergic neurons also release protein substances that can regulate the action of the transmitter, usually increasing its duration. Third, larger cortical neurons receive many excitatory and inhibitory influences from local circulatory networks and also have many different types of receptors. Activation of one type of aminergic receptor can result in a strong or weak effect depending on the initial firing state of the neuron.
Our knowledge of the physiology and pharmacodynamics of aminergic neurons is far from complete, but their importance in a wide variety of behavioral functions is beyond doubt.
 Part of a cross section through the upper part of the pons, showing elements of the reticular formation.
Part of a cross section through the upper part of the pons, showing elements of the reticular formation.