Main classes of inorganic compounds. Classification and properties of complex inorganic substances. Relationship Classification and nomenclature of complex inorganic compounds
Currently, more than 500 thousand inorganic compounds are known; it is almost impossible to know their formulas, names, and even more so their properties. In order to make it easier to navigate the huge variety of chemical substances, all substances are divided into separate classes, including compounds that are similar in structure and properties.
Initially, all chemical substances are divided into simple and complex.
Simple substances are divided into metals and non-metals.
In addition to typical metals and non-metals, there is a large group of substances with intermediate properties, they are called metalloids .
Complex substances are divided into four classes of chemical compounds: oxides, bases, acids and salts . This classification was developed by outstanding chemists of the 18th-19th centuries Antoine Laurent Lavoisier, Mikhail Vasilyevich Lomonosov, Jons Jacob Berzelius, John Dalton.
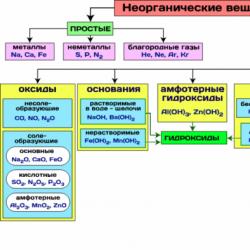
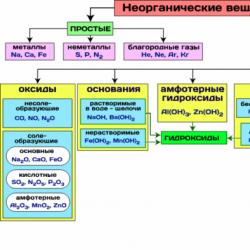
In Fig. Table 8 shows the most important classes of inorganic compounds.
Figure 8 - The most important classes of inorganic compounds
Hydroxides are a type of complex substances that contain atoms of some element E (except fluorine and oxygen) and hydroxyl groups OH; general formula of hydroxides E(OH) n, Where n= 1÷6. Form of hydroxides E(OH) n called ortho-shape; at n> 2 hydroxide can also be found in meta-form, which includes, in addition to E atoms and OH groups, oxygen atoms O, for example E(OH) 3 and EO(OH), E(OH) 4 and E(OH) 6 and EO 2 (OH) 2.
Hydroxides are divided into two groups with opposite chemical properties: acidic and basic hydroxides.
Acidic hydroxides contain hydrogen atoms, which can be replaced by metal atoms, subject to the stoichiometric valence rule. Most acid hydroxides are found in meta-form, and hydrogen atoms in the formulas of acidic hydroxides are given first place, for example, H 2 SO 4, HNO 3 and H 2 CO 3, and not SO 2 (OH) 2, NO 2 (OH) and CO (OH) 2. The general formula of acid hydroxides is H X EO at, where the electronegative component EO y x - called an acid residue. If not all hydrogen atoms are replaced by a metal, then they remain as part of the acid residue.
The names of common acid hydroxides consist of two words: the proper name with the ending “aya” and the group word “acid”.
The names of acids and acid residue are presented in table. Appendix A.
The names of acid residues are used to construct the names of salts.
Basic hydroxides contain hydroxide ions, which can be replaced by acidic residues subject to the rule of stoichiometric valence. All basic hydroxides are found in ortho-shape; their general formula is M(OH) n, Where n= 1.2 (less often 3.4) and M n+ - metal cation.
Examples of formulas and names of basic hydroxides:
The most important chemical property of basic and acidic hydroxides is their interaction with each other to form salts ( salt formation reaction), For example:
Ca(OH) 2 + H 2 SO 4 = CaSO 4 + 2H 2 O
Ca(OH) 2 + 2H 2 SO 4 = Ca(HSO 4) 2 + 2H 2 O
2Ca(OH)2 + H2SO4 = Ca2SO4(OH)2 + 2H2O
Salts- type of complex substances that contain M cations n+ and acidic residues.
Salts with general formula M X(EO at)n called average salts, and salts with unsubstituted hydrogen atoms, - sour salts. Sometimes salts also contain hydroxide and/or oxide ions; such salts are called main salts.
Here are examples and names of salts:
Acid and basic salts can be converted to middle salts by reaction with the appropriate basic and acidic hydroxide, for example:
Ca(HSO 4) 2 + Ca(OH) = CaSO 4 + 2H 2 O
Ca 2 SO 4 (OH) 2 + H 2 SO 4 = 2CaSO 4 + 2H 2 O
There are also salts containing two different cations: they are often called double salts , For example:
Acidic and basic oxides
Oxides E X ABOUT at- products of complete dehydration of hydroxides:
Acid hydroxides (H 2 SO 4, H 2 CO 3) correspond to acid oxides (SO 3, CO 2), and basic hydroxides (NaOH, Ca(OH) 2) - basic oxides (Na 2 O, CaO), and the oxidation state of element E does not change when moving from hydroxide to oxide.
Example of formulas and names of oxides:
Acidic and basic oxides retain the salt-forming properties of the corresponding hydroxides when interacting with hydroxides of opposite properties or with each other:
N 2 O 5 + 2NaOH = 2NaNO 3 + H 2 O
3CaO + 2H 3 PO 4 = Ca 3 (PO 4) 2 + 3H 2 O
La 2 O 3 + 3SO 3 = La 2 (SO 4) 3
Amphoteric oxides and hydroxides
Amphotericity hydroxides and oxides - a chemical property consisting in the formation of two rows of salts by them, for example, for aluminum hydroxide and aluminum oxide:
(a) 2Al(OH) 3 + 3SO 3 = Al 2 (SO 4) 3 + 3H 2 O
Al 2 O 3 + 3H 2 SO 4 = Al 2 (SO 4) 3 + 3H 2 O
(b) 2Al(OH) 3 + Na 2 O = 2NaAlO 2 + 3H 2 O
Al 2 O 3 + 2NaOH = 2NaAlO 2 + H 2 O
Thus, aluminum hydroxide and oxide in reactions (a) exhibit the properties main hydroxides and oxides, i.e. react with acidic hydroxides and oxide, forming the corresponding salt - aluminum sulfate Al 2 (SO 4) 3, while in reactions (b) they also exhibit the properties acidic hydroxides and oxides, i.e. react with basic hydroxide and oxide, forming a salt - sodium dioxoaluminate (III) NaAlO 2. In the first case, the element aluminum exhibits the property of a metal and is part of the electropositive component (Al 3+), in the second - the property of a non-metal and is part of the electronegative component of the salt formula (AlO 2 -).
If these reactions occur in an aqueous solution, then the composition of the resulting salts changes, but the presence of aluminum in the cation and anion remains:
2Al(OH) 3 + 3H 2 SO 4 = 2 (SO 4) 3
Al(OH) 3 + NaOH = Na
Here, complex ions 3+ - hexaaqualuminium(III) cation, - - tetrahydroxoaluminate(III) ion are highlighted in square brackets.
Elements that exhibit metallic and non-metallic properties in compounds are called amphoteric, these include elements of the A-groups of the periodic table - Be, Al, Ga, Ge, Sn, Pb, Sb, Bi, Po, etc., as well as most elements of the B- groups - Cr, Mn, Fe, Zn, Cd, Au, etc. Amphoteric oxides are called the same as basic ones, for example:
Amphoteric hydroxides (if the oxidation state of the element exceeds + II) can be found in ortho- or (and) meta- form.
Examples of amphoteric hydroxides:
Amphoteric oxides do not always correspond to amphoteric hydroxides, since when trying to obtain the latter, hydrated oxides are formed, for example:
If an amphoteric element in a compound has several oxidation states, then the amphotericity of the corresponding oxides and hydroxides (and, consequently, the amphotericity of the element itself) will be expressed differently. For low oxidation states, hydroxides and oxides have a predominance of basic properties, and the element itself has metallic properties, so it is almost always included in the composition of cations. For high oxidation states, on the contrary, hydroxides and oxides have a predominance of acidic properties, and the element itself has non-metallic properties, so it is almost always included in the composition of anions.
Salt-forming oxides:
1). Basic oxides are oxides to which bases correspond. The main oxides include oxides of metals of groups 1 and 2, as well as metals of secondary subgroups with valence I and II (except for ZnO - zinc oxide and BeO - berylium oxide): lithium oxide Li 2 O; sodium oxide Na 2 O; potassium oxide K 2 O; copper oxide CuO; silver oxide Ag2O; magnesium oxide MgO; calcium oxide CaO; strontium oxide SrO; cesium oxide Cs 2 O; mercuric oxide (2) HgO; rubidium oxide Rb 2 O; iron(2) oxide FeO; chromium oxide CrO; Nickel oxide NiO.
2). Acidic oxides are oxides that correspond to acids. Acid oxides include oxides of non-metals (except for non-salt-forming ones - indifferent), as well as oxides of metals of secondary subgroups with valence from V to VII:
carbon monoxide (IV) CO 2 ; sulfur(IV) oxide SO 2 ; sulfur(VI) oxide SO 3 ; silicon(IV) oxide SiO 2 ; phosphorus(V) oxide P 2 O 5 ; chromium(VI) dioxide CrO 3 ; manganese(VII) dioxide Mn 2 O 7 ; nitric oxide NO 2; chlorine oxides Cl 2 O 5 and Cl 2 O 3.
3). Amphoteric oxides are oxides, which correspond to bases and acids. Formed by transition metals. Metals in amphoteric oxides usually exhibit an oxidation state of +3 to +4, with the exception of ZnO, BeO, SnO, PbO: zinc oxide ZnO; chromium(III) oxide Cr 2 O 3 ; aluminum oxide Al 2 O 3 ; tin(II) oxide SnO; tin(IV) oxide SnO 2 ; lead(II) oxide PbO; lead(IV) oxide PbO 2 ; titanium(IV) oxide TiO 2 ; manganese(IV) oxide MnO 2 ; iron(III) oxide Fe 2 O 3 ; beryllium oxide BeO.
Non-salt-forming oxides
1). Non-salt-forming oxides– these are oxides indifferent to acids and bases. These include non-metal oxides with valence I and II:
carbon monoxide CO; nitric oxide (II) NO; nitric oxide (I) N 2 O; silicon(II) oxide SiO, sulfur(I) oxide S 2 O; hydrogen oxide H 2 O.
Grounds. Classification of bases
Bases are hydroxides that dissociate (break apart) into a hydroxyl group and a positively charged cation. The general formula of the bases is E(OH)m, where m is the oxidation state of the metal.
Classification of bases by strength:
1). Strong reasons.
Bases soluble in water are called alkalis:
NaOH - sodium hydroxide (caustic soda); KOH - potassium hydroxide (caustic potash); LiOH - lithium hydroxide; Ba(OH) 2 - barium hydroxide; Ca(OH) 2 - calcium hydroxide (slaked lime).
2). Weak grounds:
Mg(OH) 2 - magnesium hydroxide; Fe(OH) 2 - iron (II) hydroxide; Zn(OH) 2 - zinc hydroxide; NH 4 OH - ammonium hydroxide; A1 (OH) 3 - aluminum hydroxide; Fe(OH) 3 - iron (III) hydroxide, etc. (most metal hydroxides).
Classification of bases by solubility
A more acceptable classification of bases is based on their solubility in water.
1) Soluble bases. Alkalis- These are bases that are soluble in water. Alkalies include hydroxides of alkali and alkaline earth metals: LiOH, NaOH, KOH, RbOH, CsOH, CaOH) 2, Sr(OH) 2, Ba(OH) 2.
2). Insoluble bases- these are the so-called amphoteric hydroxides, which, when interacting with acids, act as bases, and with alkali - as acids.
Classification of bases according to the number of hydroxyl groups (OH):
1). Single acid bases (n = 1)- this is a base that contains one group - (OH): LiOH, KOH, NaOH, NH4OH.
2). Diacid bases - (n = 2)- this is a base that contains two groups - (OH): Ba(OH) 2, Mg(OH) 2, Zn(OH) 2, Fe(OH) 2.
3). Triacid bases - (n = 3)- this is a base, which includes three groups - (OH): Fe(OH) 3, A1(OH) 3, etc.
Acids. Classification of acids
Acid is a complex substance whose molecule contains one or more hydrogen atoms and an acid residue. Acids are classified according to the following criteria: a) by the presence or absence of oxygen in the molecule and b) by the number of hydrogen atoms.
a) Classification of acids according to the presence or absence of oxygen in the molecule:
1). Oxygen-containing acids: H 2 SO 4 - sulfuric acid; H 2 SO 3 - sulfurous acid; HNO 3 - nitric acid; H 3 PO 4 - phosphoric acid; H 2 CO 3 - carbonic acid; H 2 SiO 3 - silicic acid; HClO 4 - perchloric acid; HClO 3 - hydrogen trioxochlorate (V) (chloric acid); HClO 2 - hydrogen dioxochlorate(III) (chlorous acid); HClO - hydrogen oxochlorate(I) (hypochlorous acid); H 2 Cr 2 O 7 - heptaoxodichromate(VI) dihydrogen (dichromic acid); H 2 S 4 O 6 - dihydrogen hexaoxotetrasulfate (tetrathionic acid); H 2 B 4 O 6 - dihydrogen hexaoxotetraborate (tetrametaboric acid); H - hydrogen hexahydroxostibate(V); H 3 PO 3 S - thiophosphoric acid; HbSO 3 S - thiosulfuric acid; H 3 PO 3 - phosphorous (phosphonic) acid.
2). Anoxic acids: HF - hydrofluoric acid; HCl - hydrochloric acid (hydrochloric acid); HBr - hydrobromic acid; HI - hydroiodic acid; H 2 S - hydrosulfide acid; HAuCl4 - hydrogen tetrachloroaurate(III) (chlorauric acid); HSCN - hydrothiocyanate acid; HN3 - hydroazidic acid.
b) Classification of acids according to the number of hydrogen atoms:
1). Monobasic acids- these are acids that contain one ion (H +): HNO 3 - nitric acid; HF - hydrofluoric acid; HCl - hydrochloric acid; HBr - hydrobromic acid; HI - hydroiodic acid; HClO 4 - perchloric acid; HClO 3 - hydrogen trioxochlorate (V) (chloric acid); HClO 2 - hydrogen dioxochlorate(III) (chlorous acid); HClO - hydrogen oxochlorate(I) (hypochlorous acid); HAuCl 4 - hydrogen tetrachloroaurate(III) (chlorauric acid); H - hydrogen hexahydroxostibate(V); HSCN - hydrothiocyanate acid.
2). Dibasic acids- these are acids that contain two ions (H +): H 2 SO 4 - sulfuric acid; H 2 SO 3 - sulfurous acid; H 2 S - hydrosulfide acid; H 2 CO 3 - carbonic acid; H 2 SiO 3 - silicic acid; H 2 Cr 2 O 7 - heptaoxodichromate(VI) dihydrogen (dichromic acid); H 2 S 4 O 6 - dihydrogen hexaoxotetrasulfate (tetrathionic acid); H 2 B 4 O 6 - dihydrogen hexaoxotetraborate (tetrametaboric acid); H 2 SO 3 S - thiosulfuric acid.
3). Tribasic acids- these are acids that contain three ions (H +): H 3 PO 4 - phosphoric acid; H3BO3 - boric acid; H 3 AsO 4 - arsenic acid; H 3 PO 3 S - thiophosphoric acid; H 3 AlO 3 - orthoaluminum acid; H 3 PO 3 - phosphorous (phosphonic) acid.
4). Polybasic (polybasic) acids- these are acids that contain four or more ions (H +): H 4 SiO 4 - orthosilicic acid; H 4 CO 4 - orthocarbonic acid; H 4 P 2 O 7 - diphosphoric (pyrophosphoric) acid; H 6 P 6 O 18 - hexaphosphoric acid; H 6 TeO 6 - telluric acid.
Other classifications of acids:
By acid strength:
Strong acids - dissociate almost completely, dissociation constants are greater than 1 .
10 -3 (HNO 3); HCl; H 2 SO 4);
Weak acids - dissociation constant less than 1 .
10 -3 (acetic acid Kd = 1.7 .
10 -5).
In terms of stability:
Stable acids (H 2 SO 4);
Unstable acids (H 2 CO 3).
By belonging to the classes of chemical compounds:
Inorganic acids: (HBr); (H 2 SO 4);
Organic acids: (HCOOH,CH3COOH).
By volatility:
Volatile acids: (HNO 3,H 2 S);
Non-volatile acids: (H 2 SO 4).
According to solubility in water:
Soluble acids (H 2 SO 4);
Insoluble acids (H 2 SiO 3).
Salt.
Salts are substances in which metal atoms are bonded to acidic residues. The exception is ammonium salts, in which not metal atoms, but NH4+ particles are associated with acidic residues, for example, (NH4)2SO4 - ammonium sulfate.
Classification of salts:
1). Medium salts.
Medium salts- these are complex substances that dissociate in aqueous solutions into metal cations and anions of acidic residues, i.e. they are products of the replacement of all hydrogen cations in acid molecules with metal cations (Na 2 CO 3, K 3 PO 4).
2). Acidic salts.
Acid salts- these are products of partial replacement of hydrogen cations in acids with metal cations (NaHCO 3, KH 2 PO 4, K 2 HPO 4). They are formed when a base is neutralized by an excess of acid (that is, under conditions of a lack of base or an excess of acid).
3). Basic salts.
Basic salts- these are products of incomplete substitution of hydroxyl groups of the base (OH -) with acidic residues (CuOH) 2 CO 3, CoNO 3 (OH). They are formed under conditions of excess base or lack of acid.
4). Complex salts.
Complex salts- salts having complex cations or anions in which the bond is formed by a donor-acceptor mechanism. Complex ions, combining with other ions, form complex salts, for example, K 4, Cl, K 2, (Na 2), etc.
Classification of salts according to the number of cations and anions present in the structure
The following types of salts are distinguished:
1). Simple salts.
Simple salts- these are salts consisting of one type of cations and one type of anions (NaCl).
2). Double salts.
Double salts are salts containing two different types of cations. examples of double salts are (KAl(SO 4) 2 .
12H 2 O) (potassium alum), KAl(SO4) 2 (aluminum-potassium sulfate), MgK 2 (SO4) 2, AgK(CN) 2. Double salts exist only in solid form.
3). Mixed salts.
Mixed salts- these are salts that contain two different anions (Ca(OCl)Cl), Fe(NH 4) 2 (SO 4) 2 [diammonium iron(II) sulfate], LiAl(SiO 3) 2 (aluminum metasilicate- lithium), Ca(ClO)Cl (calcium chloride-hypochlorite), Na 3 CO 3 (HCO 3) (sodium bicarbonate-carbonate), Na 2 IO 3 (NO 3) (sodium nitrate-iodate)
4). Hydrate salts (crystalline hydrates).
Hydrate salts or crystalline hydrates- these are salts that contain molecules of water of crystallization, for example, Na 2 SO 4 10 H 2 O, CaSO 4 ·
2H 2 O (gipps), MgCl 2 ·
KCl ·
6H 2 O (carnallite), CuSO 4 ·
5H 2 O (copper sulfate), FeSO 4 ·
7H 2 O (iron sulfate), Na 2 CO 3 ·
10H 2 O (crystalline soda).
5). Internal salts.
Internal salts- these are salts that are formed by bipolar ions, that is, molecules containing both a positively charged and a negatively charged atom (+) NH 3 -CH 2 -COO (-) (bipolar ion of the amino acid glycine), (+) NH 3 -C 6 H 4 -SO 3 (-) (sulfanilic acid or taurine). Taurine- a sulfonic acid formed in the body from the amino acid cysteine.
The classification of inorganic substances is based on their ability to decompose. Simple substances, consisting of atoms of only one chemical element (O 2, H 2, Mg), do not disintegrate. Complex substances consisting of atoms of two or more elements (CO 2, H 2 SO 4, NaOH, KCl) easily decompose.
Simple
Classification of classes of inorganic substances includes:
- metals - elements with thermal and electrical conductivity, high ductility, malleability, and metallic luster;
- nonmetals - elements that are more fragile than metals, do not have electrical conductivity and exhibit oxidizing properties.

Rice. 1. Classification scheme for inorganic substances.
Metals are located in the lower left corner of the periodic table, nonmetals are located in the upper right corner and include the noble gases.

Rice. 2. The location of metals and non-metals in the periodic table.
Many simple chemical elements have allotropy - the property of forming several simple substances. For example, when one more atom is added to oxygen, the simple substance ozone (O 3) is formed; carbon, depending on the number of atoms, forms graphite, coal or diamond.
Complex
Complex substances are classified into the following classes:
- oxides - consist of two elements, one of which is oxygen;
- acids - consist of hydrogen atoms and an acid residue;
- grounds - consist of a metal and one or more hydroxyl groups;
- salt - consist of metal and acid residue.
Separately, amphoteric hydroxides are isolated, which exhibit the properties of acids and bases. These are solids that are weak electrolytes. These include metal hydroxides with oxidation states +3 and +4. Exceptions are Be(OH)2, Zn(OH)2, Sn(OH)2, Pb(OH)2.
A more detailed classification of complex substances is presented in the table with examples.
|
View |
Nomenclature |
Chemical properties |
Example |
|
Oxides - E x O y |
Element oxide (oxidation state) |
There are basic oxides, which form salts when interacting with acids, and acidic oxides, which form acids when interacting with bases. Separately, amphoteric oxides are isolated that interact with acids and bases (salt is formed) |
Na 2 O - sodium oxide, Fe 2 O 3 - iron (III) oxide, N 2 O 5 - nitric oxide (V) |
|
Bases - Me(OH) x |
Metal hydroxide (oxidation state) |
In accordance with solubility, alkalis and water-insoluble bases are distinguished. Alkalis react with non-metals and acid oxides. Insoluble bases react with acids and can decompose at high temperatures |
Fe(OH) 2 - iron (II) hydroxide, Cu(OH) 2 - copper (II) hydroxide, NaOH - sodium hydroxide |
|
Acids - H n Ac |
Read depending on acid residue |
They interact with metals to the left of hydrogen in the activity series, with oxides and salts. Capable of decomposition at high temperatures |
H 2 SO 4 - sulfuric acid, HCl - hydrochloric acid, HNO 3 - nitric acid |
|
Salts - Fur x (Ac) y |
Acid residue of metal (oxidation state) |
Reacts with acids, alkalis, metals and salts |
Na 2 SO 4 - sodium sulfate, CaCO 3 - calcium carbonate, KCl - potassium chloride |

Rice. 3. List of names of acids.
Genetic connections between classes are based on the mutual transformation of substances. During chemical reactions, atoms move from one substance to another, forming genetic series (series of transformations). When a metal is added to oxygen, it forms an oxide, which when reacted with water turns into a base. An acid oxide is formed from a non-metal, which reacts with water to form an acid. Any genetic series ends with salt.
What have we learned?
Inorganic substances include simple and complex compounds. Simple substances are made up of atoms of the same element. These include metals and non-metals. Complex compounds include substances consisting of several elements. These include oxides, acids, bases, salts and amphoteric hydroxides. All substances are genetically related to each other. From a simple substance you can get a more complex substance. Salts are considered the most complex substances.
Test on the topic
Evaluation of the report
Average rating: 4.6. Total ratings received: 102.
Chemical substances can be divided into two unequal groups: simple and complex.
Simple substances consist of atoms of one element (O 2, P 4).
Complex substances consist of atoms of two or more elements (CaO, H 3 PO 4).
Simple substances can be divided into metals And nonmetals.
Metals- these are simple substances in which the atoms are connected to each other by a metallic chemical bond. Metals tend to give up electrons and are characterized by metallic properties (metallic luster, high electrical and thermal conductivity, ductility, etc.).
Nonmetals – These are simple substances in which atoms are connected by covalent (or intermolecular) bonds. Nonmetals tend to accept or attract electrons. Nonmetallic properties are the ability to accept or attract electrons.
All elements in the Periodic Table of Chemical Elements (PSCE) are located either in main subgroup, or V side. In various forms of short-term PSCE, the main and secondary subgroups are located differently. There is a simple way that will allow you to quickly and reliably determine which subgroup an element belongs to. The fact is that all the elements of the second period are located in the main subgroup. Those elements that are located in the cell exactly below the elements of the second period (on the right or left) belong to the main subgroup. The rest go to the side.
For example , in the periodic table, which is used on the Unified State Exam in chemistry, element number 32, gallium, is located in the cell on the right, exactly below its corresponding second period element, boron. Therefore, gallium belongs to the main subgroup. But scandium, element number 21, is located in the cell on the left. Therefore, scandium belongs to the secondary subgroup.
Nonmetals are located in main subgroups, in the upper right corner of the PSHE. All metals include elements of side subgroups And elements of the main subgroups located in the lower left part of the PSHE. Metals and non-metals are usually separated by drawing a conventional line from beryllium to astatine. The figure shows the precise division into metals and non-metals. Non-metals are colored.
The main classes of complex substances are oxides, hydroxides, salt.
Oxides- these are complex substances that consist of atoms of two elements, one of which is oxygen, which has an oxidation state of -2.
Depending on the second element, oxides exhibit different chemical properties. Some oxides correspond to hydroxides (salt-forming oxides), and some do not (non-salt-forming oxides).
Salt-forming oxides divided into basic, amphoteric and acidic.
Basic oxides are oxides that exhibit characteristic basic properties. These include oxides formed by atoms metals with oxidation state +1 and +2 . For example, lithium oxide Li 2 O, iron oxide (II) FeO.
Acidic oxides are oxides that exhibit acidic properties. These include oxides formed by atoms metals with oxidation states +5, +6 and +7 , as well as non-metal atoms with any degree of oxidation . For example, chlorine oxide (I) Cl 2 O, chromium oxide (VI) CrO 3.
Amphoteric oxides are oxides that exhibit both basic and acidic properties. These are oxides metals with oxidation state +3 and +4 , as well as four oxides with oxidation state +2: ZnO, PbO, SnO and BeO .
Non-salt-forming oxides do not exhibit characteristic basic or acidic properties; hydroxides do not correspond to them. Four oxides are classified as non-salt-forming: CO, NO, N 2 O and SiO .
There are also oxides similar to salts, i.e. salt-like (double).
Double oxides - These are some oxides formed by an element with different oxidation states. For example, magnetite (magnetic iron ore) FeO·Fe 2 O 3.

Algorithm for determining the oxide type: at first determine which element forms the oxide – metal or non-metal. If it is a metal, then we determine the oxidation state, then determine the type of oxide. If it is a non-metal, then the oxide is acidic (unless this is an exception).
Hydroxides- these are complex substances that contain the E-O-H group. Hydroxides include bases, amphoteric hydroxides, and oxygen-containing acids.
Each salt-forming oxide corresponds to a hydroxide:
basic oxide corresponds to hydroxide base ,
acid oxide corresponds to hydroxide acid ,
amphoteric oxide corresponds amphoteric hydroxide .
For example, chromium (II) oxide CrO is basic; it corresponds to a hydroxide base. The hydroxide formula is easy to obtain by simply adding the hydroxide group OH to the metal: Cr(OH) 2 .
Chromium (VI) oxide is acidic, it corresponds to the hydroxide acid H 2 CrO 4, and the acidic residue is the chromate ion CrO 4 2-.
If all indices are multiples of 2, then we divide all indices by 2.
For example: N 2 O 5 + H 2 O → H 2 N 2 O 6, divide by 2, we get HNO 3. So we get meta-formula acids. If we add one more water molecule, we get ortho formula acids.
For example: oxide P 2 O 5 , meta-form: HPO 3 . Add water, ortho form: H 3 PO 4. The ortho form is stable against phosphorus and arsenic.
Chromium (III) oxide - Cr 2 O 3 - is amphoteric, it corresponds to an amphoteric hydroxide, which can act both as a base and as an acid: Cr(OH) 3 = HCrO 2, acidic residue chromite: CrO 2 -.
The relationship between oxides and hydroxides:
Reasons(basic hydroxides) are complex substances that, when dissociated in aqueous solutions as anions (negative ions), form only hydroxide ions OH - .
Bases can be divided into those that are soluble in water ( alkalis ), insolublein water, and spontaneouslydecomposing .
TO decomposes in water (unstable) bases include ammonium hydroxide, silver (I) hydroxide, copper (I) hydroxide. In an aqueous solution, such compounds decompose almost irreversibly:
NH 4 OH → NH 3 + H 2 O
2AgOH → Ag 2 O + H 2 O
2CuOH → Cu 2 O + H 2 O
Bases with one OH group – monoacid(For example, NaOH), with two - diacid(Ca(OH)2) and with three - triacid(Fe(OH) 3).
Acids- these are complex substances that, when dissociated in aqueous solutions, form only hydronium ions H 3 O + (H +) as cations. Acids consist of hydrogen H+ and an acid residue.
According to the number of hydrogen atoms that can be replaced by metals, acids are divided into monobasic (HNO3), dibasic(H 2 SO 4), tribasic (H 3 PO 4), etc.
Acids can also be divided into strong and weak.
Strong acids. These include:
- Anoxic acids: HCl, HBr, HI. The remaining oxygen-free acids are usually weak.
- Some higher oxygen-containing acids: H2SO4, HNO3, HClO4 and etc.
Weak acids . These include:
- Weak and soluble acids : This H3PO4, CH3COOH, HF and etc.
- Volatile or unstable acids : H2S— gas; H2CO3 H 2 CO 3 → H 2 O + CO 2; H2SO3- decomposes into water and oxide: H 2 SO 3 → H 2 O+ SO 2.
- Water-insoluble acids : H2SiO3, H3BO3 and others.
A simple technique allows you to determine whether the acid in front of you is strong or weak. We subtract the number of H atoms from the number of O atoms in the acid. If we get the number 2 or 3, then the acid strong. If 1 or 0 - then acid weak.
Salts– complex substances consisting of a metal cation (or metal-like cations, for example, ammonium ion NH 4 +) and an acid residue anion. Salts are also called substances that can be obtained by the interaction of acids and bases with the release of water.
If we consider salts as acid-base reaction products, then the salts are divided into average , sour And basic .
Average salt – products of complete replacement of hydrogen cations in acid with metal cations ( For example , Na 2 CO 3 , K 3 PO 4).
Sour salt – products of incomplete replacement of hydrogen cations in acid with metal cations ( For example , NaHCO3, K2HPO4).
Basic salt – products of incomplete substitution of hydroxyl groups of the base with anions of acidic acid residues ( For example, malachite (CuOH)2CO3).
According to the number of cations and anions salts are divided into:
Simple salts – consisting of a cation of one type and anion of one type ( For example, calcium chloride CaCl2).
Double salts are salts consisting of two or more different cations and an anion of the same type ( For example, potassium alum – KAl(SO 4) 2).
Mixed salts are salts consisting of a cation of one type and two or more anions of different types ( For example, calcium chloride-hypochlorite Ca(OCl)Cl).
Based on structural features, they also distinguish hydrate salt and complex salt.
Hydrate salts (crystal hydrates) - these are salts that contain molecules of water of crystallization ( For example, sodium sulfate decahydrate Na 2 SO 4 10 H 2 O).
Complex salts are salts containing a complex cation or complex anion ( K3, (OH)2).
In addition to the main classes of inorganic compounds, there are a large number of others. For example, binary compounds of elements with hydrogen.
Hydrogen compounds are complex substances consisting of two elements, one of which is hydrogen. Hydrogen forms salt-like hydrides and volatile hydrogen compounds.
Salt-like hydrides EN x are compounds of group IA, IIA metals and aluminum with hydrogen. The oxidation state of hydrogen is -1. For example, sodium hydride NaH.
Volatile hydrogen compounds H x E are compounds of non-metals with hydrogen in which the oxidation state of hydrogen is +1. For example, ammonia NH 3, phosphine PH 3.
Chemistry studies the transformations of chemical substances, of which more than 20 million are known today. Therefore, the classification of chemical compounds is important, i.e., combining them into groups or classes that have similar properties. This lesson will help you study the modern classification of inorganic substances and introduce you to the rules for compiling their names using chemical formulas.
Topic: Main classes of compounds, their properties and typical reactions
Lesson: Classification and nomenclature of inorganic substances
Inorganic substances are usually divided into two groups according to their composition: a small group of simple substances (there are about 400 of them) and a very large group of complex substances. Simple substances consist of one chemical element, while complex substances consist of several.
All simple substances can be divided into metals and non-metals, since their properties differ significantly. Metals have a metallic luster, high thermal and electrical conductivity, are ductile, and exhibit restorative properties. Nonmetals have very different physical and chemical properties, but, as a rule, in the solid state they are brittle and do not conduct electricity and heat well.
The boundary between metals and non-metals is arbitrary. There are substances that have the properties of both metals and non-metals. For example, gray arsenic has a metallic luster and electrical conductivity (Fig. 1), while another allotropic modification - yellow arsenic - has purely non-metallic properties.
Rice. 1. Gray arsenic
Complex substances are usually divided into classes: oxides, acids, bases, amphoteric hydroxides and salts (Figure 2). This classification is imperfect, because there is no place in it for ammonia, metal compounds with phosphorus, nitrogen, carbon, etc.

Rice. 2. Classification of inorganic substances
Oxides can be salt-forming or non-salt-forming. Salt-forming oxides correspond to hydroxides and salts with an element in the same oxidation state as in the oxide. Non-salt-forming oxides do not have corresponding hydroxides and salts. There are few such oxides: N 2 O, NO, SiO, CO.
Salt-forming oxides, depending on their acid-base character, are divided into acidic, amphoteric and basic.
Basic oxides are formed by metals with small oxidation states +1, +2. Amphoteric oxides are formed by transition metals with oxidation states +3, +4, as well as Be, Zn, Sn, Pb. Acidic oxides are formed by non-metals, as well as metals with an oxidation state greater than +4. Rice. 3.

Rice. 3. Classification of oxides
Acids are complex substances consisting of hydrogen atoms that can be replaced by metals and acidic residues. Acids can be divided into groups according to oxygen content: oxygen-containing (for example, HNO 3, H 2 SO 4, H 3 PO 4) and oxygen-free (HI, H 2 S). Rice. 4.

Rice. 4. Classification of acids
Bases are complex substances consisting of metal cations and one or more hydroxide anions. The classification of bases can be based on different characteristics. For example, their relationship to water. Based on this criterion, bases are divided into water-soluble (alkalis) and water-insoluble. Rice. 5.

Rice. 5. Classification of bases
Amphoteric hydroxides are complex substances that have the properties of both acids and bases, and therefore their formulas can be written in different forms:
Zn(OH) 2 = H 2 ZnO 2
base form acid form
There are several types of salts (Fig. 6).

Rice. 6. Types of salts
Medium salts consist of metal (or ammonium) cations and anions of acidic residues. Acidic salts, in addition to metal cations, contain hydrogen cations and an anion of an acid residue. Basic salts contain hydroxide anions.
If a salt is formed by two types of metal cations and one anion, then it is called double. For example, aluminum-potassium sulfate KAl(SO 4) 2.
Salts with two different anions and one cation are called mixed. For example, Ca(OCl)Cl is calcium chloride-hypochlorite.
Complex salts contain a complex ion, which is usually enclosed in square brackets.
Bibliography
- Kuznetsova N.E., Litvinova T.N., Levkin A.N. Chemistry: 11th grade: textbook for general education students. establishment (profile level): in 2 parts. Part 2. - M.: Ventana-Graf, 2008. (§55)
- Radetsky A.M. Chemistry. Didactic material. 10-11 grades. - M.: Education, 2011.
- Khomchenko I.D. Collection of problems and exercises in chemistry for high school. - M.: RIA “New Wave”: Publisher Umerenkov, 2008. (p. 27-30)
- Encyclopedia for children. Volume 17. Chemistry / Chapter. ed. V.A. Volodin, Ved. scientific ed. I. Leenson. - M.: Avanta+, 2003. (p. 156-159)