Molecular formula h2so4. Sulfuric acid structural chemical formula. Interaction with non-metals
Undiluted sulfuric acid is a covalent compound.
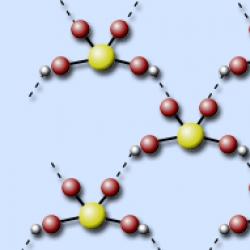
In the molecule, sulfuric acid is tetrahedrally surrounded by four oxygen atoms, two of which are part of the hydroxyl groups. The S–O bonds are double, and the S–OH bonds are single.
The colorless, ice-like crystals have a layered structure: each H 2 SO 4 molecule is connected to four neighboring strong hydrogen bonds, forming a single spatial framework.

The structure of liquid sulfuric acid is similar to the structure of solid one, only the integrity of the spatial framework is broken.
Physical properties of sulfuric acid
Under normal conditions, sulfuric acid is a heavy, oily liquid without color or odor. In technology, sulfuric acid is a mixture of both water and sulfuric anhydride. If the molar ratio of SO 3: H 2 O is less than 1, then it is an aqueous solution of sulfuric acid; if it is greater than 1, it is a solution of SO 3 in sulfuric acid.
100% H 2 SO 4 crystallizes at 10.45 ° C; T kip = 296.2 °C; density 1.98 g/cm3. H 2 SO 4 mixes with H 2 O and SO 3 in any ratio to form hydrates; the heat of hydration is so high that the mixture can boil, splash and cause burns. Therefore, it is necessary to add acid to water, and not vice versa, since when water is added to acid, lighter water will end up on the surface of the acid, where all the heat generated will be concentrated.
When aqueous solutions of sulfuric acid containing up to 70% H 2 SO 4 are heated and boiled, only water vapor is released into the vapor phase. Sulfuric acid vapor also appears above more concentrated solutions.
In terms of structural features and anomalies, liquid sulfuric acid is similar to water. There is the same system of hydrogen bonds, almost the same spatial framework.
Chemical properties of sulfuric acid
Sulfuric acid is one of the strongest mineral acids; due to its high polarity, the H–O bond is easily broken.
Sulfuric acid dissociates in aqueous solution , forming a hydrogen ion and an acidic residue:
H 2 SO 4 = H + + HSO 4 - ;
HSO 4 - = H + + SO 4 2- .
Summary equation:
H 2 SO 4 = 2H + + SO 4 2- .
Shows properties of acids , reacts with metals, metal oxides, bases and salts.
Dilute sulfuric acid does not exhibit oxidizing properties; when it interacts with metals, hydrogen and a salt containing the metal in the lowest oxidation state are released. In the cold, the acid is inert towards metals such as iron, aluminum and even barium.
Concentrated acid has oxidizing properties. Possible products of the interaction of simple substances with concentrated sulfuric acid are given in the table. The dependence of the reduction product on the acid concentration and the degree of activity of the metal is shown: the more active the metal, the more deeply it reduces the sulfate ion of sulfuric acid.

Interaction with oxides:
CaO + H 2 SO 4 = CaSO 4 = H 2 O.
Interaction with bases:
2NaOH + H 2 SO 4 = Na 2 SO 4 + 2H 2 O.
Interaction with salts:
Na 2 CO 3 + H 2 SO 4 = Na 2 SO 4 + CO 2 + H 2 O.
Oxidative properties
Sulfuric acid oxidizes HI and HBr to free halogens:
H 2 SO 4 + 2HI = I 2 + 2H 2 O + SO 2.
Sulfuric acid removes chemically bound water from organic compounds containing hydroxyl groups. Dehydration of ethyl alcohol in the presence of concentrated sulfuric acid leads to the production of ethylene:
C 2 H 5 OH = C 2 H 4 + H 2 O.
The charring of sugar, cellulose, starch and other carbohydrates upon contact with sulfuric acid is also explained by their dehydration:
C 6 H 12 O 6 + 12H 2 SO 4 = 18H 2 O + 12SO 2 + 6CO 2.
Acids are chemical compounds consisting of hydrogen atoms and acidic residues, for example, SO4, SO3, PO4, etc. They are inorganic and organic. The first include hydrochloric, phosphoric, sulfide, nitric, and sulfuric acid. The second ones include acetic acid, palmitic acid, formic acid, stearic acid, etc.
What is sulfuric acid
This acid consists of two hydrogen atoms and the acidic residue SO4. It has the formula H2SO4.
Sulfuric acid or, as it is also called, sulfate acid, refers to inorganic oxygen-containing dibasic acids. This substance is considered one of the most aggressive and chemically active. In most chemical reactions it acts as an oxidizing agent. This acid can be used in concentrated or dilute form, in which case it has slightly different chemical properties.
Physical properties
Sulfuric acid under normal conditions is liquid, its boiling point is approximately 279.6 degrees Celsius, the freezing point when it turns into solid crystals is about -10 degrees for one hundred percent and about -20 for 95 percent.

Pure one hundred percent sulfate acid is an odorless, colorless, oily liquid substance that has almost twice the density of water - 1840 kg/m3.
Chemical properties of sulfate acid
Sulfuric acid reacts with metals, their oxides, hydroxides and salts. Diluted with water in different proportions, it can behave differently, so let’s take a closer look at the properties of concentrated and weak solutions of sulfuric acid separately.
Concentrated sulfuric acid solution
A solution containing at least 90 percent sulfate acid is considered concentrated. Such a solution of sulfuric acid is capable of reacting even with low-active metals, as well as non-metals, hydroxides, oxides, and salts. The properties of such a solution of sulfate acid are similar to those of concentrated nitrate acid.

Interaction with metals
During the chemical reaction of a concentrated solution of sulfate acid with metals located to the right of hydrogen in the electrochemical voltage series of metals (that is, with not the most active ones), the following substances are formed: sulfate of the metal with which the interaction occurs, water and sulfur dioxide. Metals, as a result of interaction with which the listed substances are formed, include copper (cuprum), mercury, bismuth, silver (argentum), platinum and gold (aurum).

Interaction with inactive metals
With metals that are to the left of hydrogen in the voltage series, concentrated sulfuric acid behaves slightly differently. As a result of this chemical reaction, the following substances are formed: sulfate of a certain metal, hydrogen sulfide or pure sulfur and water. The metals with which a similar reaction occurs also include iron (ferum), magnesium, manganese, beryllium, lithium, barium, calcium and all others that are in the voltage series to the left of hydrogen, except aluminum, chromium, nickel and titanium - with them concentrated sulfate acid does not interact.
Interaction with non-metals
This substance is a strong oxidizing agent, so it is capable of participating in redox chemical reactions with non-metals, such as, for example, carbon (carbon) and sulfur. As a result of such reactions, water is necessarily released. When this substance is added to carbon, carbon dioxide and sulfur dioxide are also released. And if you add acid to sulfur, you get only sulfur dioxide and water. In such a chemical reaction, sulfate acid plays the role of an oxidizing agent.
Interaction with organic substances
Among the reactions of sulfuric acid with organic substances, charring can be distinguished. This process occurs when this substance collides with paper, sugar, fibers, wood, etc. In this case, carbon is released in any case. The carbon formed during the reaction can partially react with sulfuric acid if it is in excess. The photo shows the reaction of sugar with a solution of sulfate acid of medium concentration.

Reactions with salts
Also, a concentrated solution of H2SO4 reacts with dry salts. In this case, a standard exchange reaction occurs, in which the metal sulfate that was present in the salt structure and the acid with the residue that was in the salt are formed. However, concentrated sulfuric acid does not react with salt solutions.
Interaction with other substances
Also, this substance can react with metal oxides and their hydroxides, in these cases exchange reactions occur, in the first, metal sulfate and water are released, in the second - the same.
Chemical properties of a weak solution of sulfate acid
Dilute sulfuric acid reacts with many substances and has the same properties as all acids. It, unlike concentrated metal, interacts only with active metals, that is, those that are to the left of hydrogen in the voltage series. In this case, the same substitution reaction occurs as in the case of any acid. This releases hydrogen. Also, such an acid solution interacts with salt solutions, resulting in an exchange reaction, already discussed above, with oxides - the same as a concentrated one, with hydroxides - also the same. In addition to ordinary sulfates, there are also hydrosulfates, which are the product of the interaction of hydroxide and sulfuric acid.

How to tell if a solution contains sulfuric acid or sulfates
To determine whether these substances are present in a solution, a special qualitative reaction to sulfate ions is used, which makes it possible to find out. It consists of adding barium or its compounds to the solution. This may result in a white precipitate (barium sulfate), indicating the presence of sulfates or sulfuric acid.
How is sulfuric acid produced?
The most common method of industrial production of this substance is its extraction from iron pyrite. This process occurs in three stages, each of which involves a specific chemical reaction. Let's look at them. First, oxygen is added to pyrite, resulting in the formation of ferum oxide and sulfur dioxide, which is used for further reactions. This interaction occurs at high temperature. Next comes the stage in which sulfur trioxide is obtained by adding oxygen in the presence of a catalyst, which is vanadium oxide. Now, at the last stage, water is added to the resulting substance, and sulfate acid is obtained. This is the most common process for the industrial extraction of sulfate acid, it is used most often because pyrite is the most accessible raw material suitable for the synthesis of the substance described in this article. Sulfuric acid obtained through this process is used in various fields of industry - both in the chemical and in many others, for example, in oil refining, ore dressing, etc. Its use is also often provided for in the manufacturing technology of many synthetic fibers .
Target: Familiarize yourself with the structure, physical and chemical properties, and use of sulfuric acid.
Educational objectives: Consider the physical and chemical properties (common with other acids and specific) of sulfuric acid, preparation, show the great importance of sulfuric acid and its salts in the national economy.
Educational tasks: Continue to develop in students a dialectical-materialistic understanding of nature.
Developmental tasks: Development of general educational skills and abilities, working with a textbook and additional literature, rules for working on the desktop, the ability to systematize and generalize, establish cause-and-effect relationships, express one’s thoughts conclusively and competently, draw conclusions, draw up diagrams, sketch.
During the classes
1. Repetition of what has been covered.
Frontal class survey. Compare the properties of crystalline and plastic sulfur. Explain the essence of allotropy.
2. Studying new material.
After listening to the story carefully, at the end of the lesson we will explain why sulfuric acid behaved strangely with water, wood and a gold ring.
An audio recording plays.
The Adventures of Sulfuric Acid.
In one chemical kingdom there lived a sorceress, her name was Sulfuric acid. In appearance it was not so bad: a colorless liquid, viscous like oil, odorless. Sulfuric acid I wanted to be famous, so I went on a trip.
She had been walking for 5 hours, and since the day was too hot, she was very thirsty. And suddenly she saw a well. "Water!" - exclaimed the acid and, running up to the well, touched the water. The water hissed terribly. With a scream, the frightened sorceress rushed away. Of course, the young acid did not know that when mixed sulfuric acid A large amount of heat is released with water.
"If water comes into contact with sulfuric acid, then the water, not having time to mix with the acid, can boil and throw out splashes sulfuric acid. This entry appeared in the diary of a young traveler, and then entered textbooks.
Since the acid did not quench her thirst, the spreading tree decided to lie down and rest in the shade. But she didn’t succeed in that either. As soon as Sulfuric acid I touched the wood, it began to char. Not knowing the reason for this, the frightened acid ran away.
Soon she came to the city and decided to go to the first store she came across on her way. It turned out to be a jewelry store. Approaching the display cases, the acid saw many beautiful rings. Sulfuric acid I decided to try on one ring. Having asked the seller for a gold ring, the traveler put it on her long, beautiful finger. The sorceress really liked the ring and decided to buy it. This is what she could brag about to her friends!
After leaving the city, the acid went home. On the way, she was haunted by the thought of why water and wood behaved so strangely when touching her, but nothing happened to this golden thing? “Yes, because gold is in sulfuric acid does not oxidize." These were the last words he wrote with acid in his diary.
Teacher's explanations.
Electronic and structural formulas of sulfuric acid.
Since sulfur is in the 3rd period of the periodic table, the octet rule (eight electron structure) is not observed and the sulfur atom can acquire up to twelve electrons. The electronic and structural formulas of sulfuric acid are as follows:
(Sulfur's six electrons are indicated by an asterisk)
Receipt.
Sulfuric acid is formed by the interaction of sulfur oxide (5) with water (SO 3 + H 2 O -> H 2 SO 4).
Physical properties.
Sulfuric acid is a colorless, heavy, non-volatile liquid. When it is dissolved in water, very strong heating occurs. remember, that Do not pour water into concentrated sulfuric acid!
Concentrated sulfuric acid absorbs water vapor from the air. This can be verified if an open vessel with concentrated sulfuric acid is balanced on a scale: after some time the cup with the vessel will drop.
Chemical properties.
Dilute sulfuric acid has common properties characteristic of all acids. In addition, sulfuric acid has specific properties.
Chemical properties of sulfur - Application .
Teacher demonstration of an entertaining experience.
Brief safety briefing.
Popsicle (Coal from Sugar)
| Equipment | Experience Plan | Conclusion |
|
Pour 30 g of powdered sugar into a beaker. Measure 12 ml of concentrated sulfuric acid using a beaker. Mix sugar and acid in a glass with a glass rod into a porridge-like mass (remove the glass rod and place in a glass of water). After some time, the mixture darkens, warms up, and soon a porous coal mass begins to crawl out of the glass - popsicle | The charring of sugar by sulfuric acid (concentrated) is explained by the oxidizing properties of this acid. The reducing agent is carbon. The process is exothermic. 2H 2 SO 4 +C 12 O 11 + H22 -> 11C + 2SO 2 +13H 2 O + CO 2 |
Students fill out a table with entertaining experiences in their notebooks.
Students' reasoning about why Sulfuric acid behaved so strangely with water, wood and gold.
Application.
Due to its properties (the ability to absorb water, oxidizing properties, non-volatility), sulfuric acid is widely used in the national economy. It belongs to the main products of the chemical industry.

- obtaining dyes;
- obtaining mineral fertilizers;
- petroleum products purification;
- electrolytic copper production;
- electrolyte in batteries;
- obtaining explosives;
- obtaining dyes;
- obtaining artificial silk;
- obtaining glucose;
- obtaining salts;
- production of acids.
Sulfuric acid salts are widely used, for example
Na 2 SO 4 * 10H 2 O– sodium sulfate crystalline hydrate (Glauber's salt)- used in the production of soda, glass, medicine and veterinary medicine.
CaSO 4 * 2H 2 O– calcium sulfate crystalline hydrate (natural gypsum)- used to obtain semi-aqueous gypsum, necessary in construction, and in medicine - for applying plaster casts.
CuSO 4 * 5H 2 O– copper sulfate crystalline hydrate (2) (copper sulfate)- used in the fight against pests and plant diseases.
Students' work with the extra-textual component of the textbook.
This is interesting
...in the Kara-Bogaz-Gol Bay, the water contains 30% Glauber's salt at a temperature of +5°C, this salt falls out in the form of a white sediment, like snow, and with the onset of warm weather, the salt dissolves again. Since Glauber's salt appears and disappears in this bay, it was named mirabilite, which means “amazing salt.”
3. Questions to reinforce educational material, written on the board.
- In winter, a vessel with concentrated sulfuric acid is sometimes placed between the window frames. For what purpose is this done, why can’t the vessel be filled to the top with acid?
- Why is sulfuric acid called the “bread” of chemistry?
Homework and instructions on how to complete it.

Where required, write equations in ionic form.
Conclusion on the lesson, marking and commenting.
References.
- Rudzitis G.E. Feldman F.G., Chemistry: Textbook for grades 7-11 of evening (shift) secondary school at 2 o'clock. Part 1-3 edition - M.: Prosveshchenie, 1987.
- Chemistry at school No. 6, 1991.
- Strempler Genrikh Ivanovich, Chemistry at leisure: Book. for middle school students and old age /Fig. auto with the participation of V.N. Rastopchiny.- F.: Ch. ed. KSE, 1990.
Structural formula
True, empirical, or gross formula: H2SO4
Chemical composition of Sulfuric acid
Molecular weight: 98.076
Sulfuric acid H 2 SO 4 is a strong dibasic acid corresponding to the highest oxidation state of sulfur (+6). Under normal conditions, concentrated sulfuric acid is a heavy, oily liquid, colorless and odorless, with a sour “copper” taste. In technology, sulfuric acid is called its mixture with both water and sulfuric anhydride SO 3. If the molar ratio of SO 3: H 2 O is less than 1, then it is an aqueous solution of sulfuric acid; if it is greater than 1, it is a solution of SO 3 in sulfuric acid (oleum).
Name
In the 18th-19th centuries, sulfur for gunpowder was produced from sulfur pyrite (pyrite) in vitriol factories. Sulfuric acid at that time was called “oil of vitriol” (as a rule, it was a crystalline hydrate, with a consistency reminiscent of oil), obviously hence the origin of the name of its salts (or rather, crystalline hydrates) - vitriol.
Preparation of sulfuric acid
Industrial (contact) method
In industry, sulfuric acid is produced by the oxidation of sulfur dioxide (sulfur dioxide gas formed during the combustion of sulfur or sulfur pyrites) to trioxide (sulfuric anhydride), followed by the reaction of SO 3 with water. The sulfuric acid obtained by this method is also called contact acid (concentration 92-94%).
Nitrose (tower) method
Previously, sulfuric acid was produced exclusively by the nitrous method in special towers, and the acid was called tower acid (concentration 75%). The essence of this method is the oxidation of sulfur dioxide with nitrogen dioxide in the presence of water.
Another way
In those rare cases when hydrogen sulfide (H 2 S) displaces sulfate (SO 4 -) from the salt (with the metals Cu, Ag, Pb, Hg), the by-product is sulfuric acid. Sulfides of these metals have the highest strength, as well as a distinctive black color.
Physical and physico-chemical properties
A very strong acid, at 18 o C pK a (1) = −2.8, pK a (2) = 1.92 (K z 1.2 10 -2); bond lengths in the molecule S=O 0.143 nm, S-OH 0.154 nm, HOSOH angle 104°, OSO 119°; boils, forming an azeotropic mixture (98.3% H 2 SO 4 and 1.7% H 2 O with a boiling point of 338.8 o C). Sulfuric acid corresponding to 100% H 2 SO 4 content has the composition (%): H 2 SO 4 99.5, HSO 4 - - 0.18, H 3 SO 4 + - 0.14, H 3 O + - 0.09, H 2 S 2 O 7, - 0.04, HS 2 O 7 - - 0.05. Miscible with water and SO 3 in all proportions. In aqueous solutions, sulfuric acid almost completely dissociates into H 3 O +, HSO 3 +, and 2HSO 4 -. Forms hydrates H 2 SO 4 ·nH 2 O, where n = 1, 2, 3, 4 and 6.5.
Oleum
Solutions of sulfuric anhydride SO 3 in sulfuric acid are called oleum; they form two compounds H 2 SO 4 ·SO 3 and H 2 SO 4 ·2SO 3. Oleum also contains pyrosulfuric acids. The boiling point of aqueous solutions of sulfuric acid increases with increasing its concentration and reaches a maximum at a content of 98.3% H 2 SO 4. The boiling point of oleum decreases with increasing SO3 content. As the concentration of aqueous solutions of sulfuric acid increases, the total vapor pressure above the solutions decreases and reaches a minimum at a content of 98.3% H 2 SO 4. As the concentration of SO 3 in oleum increases, the total vapor pressure above it increases. The vapor pressure over aqueous solutions of sulfuric acid and oleum can be calculated using the equation:
log p=A-B/T+2.126
the values of coefficients A and B depend on the concentration of sulfuric acid. Steam over aqueous solutions of sulfuric acid consists of a mixture of water vapor, H 2 SO 4 and SO 3, and the composition of the vapor differs from the composition of the liquid at all concentrations of sulfuric acid, except for the corresponding azeotropic mixture. As temperature increases, dissociation increases. Oleum H2SO4·SO3 has the maximum viscosity; with increasing temperature, η decreases. The electrical resistance of sulfuric acid is minimal at a concentration of SO 3 and 92% H 2 SO 4 and maximum at a concentration of 84 and 99.8% H 2 SO 4. For oleum, the minimum ρ is at a concentration of 10% SO 3. With increasing temperature, ρ of sulfuric acid increases. Dielectric constant of 100% sulfuric acid 101 (298.15 K), 122 (281.15 K); cryoscopic constant 6.12, ebullioscopic constant 5.33; the diffusion coefficient of sulfuric acid vapor in air varies depending on temperature; D = 1.67·10⁻⁵T3/2 cm²/s.
Chemical properties
Sulfuric acid in concentrated form when heated is a fairly strong oxidizing agent. Oxidizes HI and partially HBr to free halogens. Oxidizes many metals (exceptions: Au, Pt, Ir, Rh, Ta.). In this case, concentrated sulfuric acid is reduced to SO 2. In the cold in concentrated sulfuric acid, Fe, Al, Cr, Co, Ni, Ba are passivated and reactions do not occur. The most powerful reducing agents reduce concentrated sulfuric acid to S and H 2 S. Concentrated sulfuric acid absorbs water vapor, so it is used for drying gases, liquids and solids, for example, in desiccators. However, concentrated H 2 SO 4 is partially reduced by hydrogen, which is why it cannot be used for drying it. By splitting water from organic compounds and leaving behind black carbon (charcoal), concentrated sulfuric acid leads to charring of wood, sugar and other substances. Dilute H 2 SO 4 interacts with all metals located in the electrochemical voltage series to the left of hydrogen with its release. The oxidizing properties of dilute H 2 SO 4 are uncharacteristic. Sulfuric acid forms two series of salts: medium - sulfates and acidic - hydrosulfates, as well as esters. Peroxomonosulfuric (or Caro acid) H 2 SO 5 and peroxodisulfuric H 2 S 2 O 8 acids are known. Sulfuric acid also reacts with basic oxides to form sulfate and water. In metalworking plants, a solution of sulfuric acid is used to remove a layer of metal oxide from the surface of metal products that are subjected to high heat during the manufacturing process. Thus, iron oxide is removed from the surface of sheet iron by the action of a heated solution of sulfuric acid. A qualitative reaction to sulfuric acid and its soluble salts is their interaction with soluble barium salts, which results in the formation of a white precipitate of barium sulfate, insoluble in water and acids, for example.
Application
Sulfuric acid is used:
- in ore processing, especially in the extraction of rare elements, including uranium, iridium, zirconium, osmium, etc.;
- in the production of mineral fertilizers;
- as an electrolyte in lead batteries;
- for obtaining various mineral acids and salts;
- in the production of chemical fibers, dyes, smoke-forming and explosives;
- in the oil, metalworking, textile, leather and other industries;
- in the food industry - registered as food additive E513 (emulsifier);
- in industrial organic synthesis in reactions:
- dehydration (production of diethyl ether, esters);
- hydration (ethanol from ethylene);
- sulfonation (synthetic detergents and intermediates in the production of dyes);
- alkylation (production of isooctane, polyethylene glycol, caprolactam), etc.
- For the restoration of resins in filters in the production of distilled water.
World production of sulfuric acid is approx. 160 million tons per year. The largest consumer of sulfuric acid is the production of mineral fertilizers. P 2 O 5 phosphorus fertilizers consume 2.2-3.4 times more mass of sulfuric acid, and (NH 4) 2 SO 4 sulfuric acid consumes 75% of the mass of consumed (NH 4) 2 SO 4. Therefore, they tend to build sulfuric acid plants in conjunction with factories for the production of mineral fertilizers.
Historical information
Sulfuric acid has been known since ancient times, occurring in nature in free form, for example, in the form of lakes near volcanoes. Perhaps the first mention of acid gases produced by the calcination of alum or iron sulfate of the “green stone” is found in writings attributed to the Arab alchemist Jabir ibn Hayyan. In the 9th century, the Persian alchemist Ar-Razi, calcining a mixture of iron and copper sulfate (FeSO 4 7H 2 O and CuSO 4 5H 2 O), also obtained a solution of sulfuric acid. This method was improved by the European alchemist Albert Magnus, who lived in the 13th century. The scheme for producing sulfuric acid from ferrous sulfate is the thermal decomposition of iron (II) sulfate followed by cooling of the mixture. The works of the alchemist Valentin (13th century) describe a method for producing sulfuric acid by absorbing gas (sulfuric anhydride) released by burning a mixture of sulfur and nitrate powders with water. Subsequently, this method formed the basis of the so-called. “chamber” method, carried out in small chambers lined with lead, which does not dissolve in sulfuric acid. In the USSR, this method existed until 1955. Alchemists of the 15th century also knew a method for producing sulfuric acid from pyrite - sulfur pyrite, a cheaper and more common raw material than sulfur. Sulfuric acid has been produced in this way for 300 years, in small quantities in glass retorts. Subsequently, in connection with the development of catalysis, this method replaced the chamber method for the synthesis of sulfuric acid. Currently, sulfuric acid is produced by the catalytic oxidation (on V 2 O 5) of sulfur oxide (IV) to sulfur oxide (VI), and subsequent dissolution of sulfur oxide (VI) in 70% sulfuric acid to form oleum. In Russia, the production of sulfuric acid was first organized in 1805 near Moscow in the Zvenigorod district. In 1913, Russia ranked 13th in the world in sulfuric acid production.
additional information
Tiny droplets of sulfuric acid can form in the middle and upper layers of the atmosphere as a result of the reaction of water vapor and volcanic ash containing large quantities of sulfur. The resulting suspension, due to the high albedo of sulfuric acid clouds, makes it difficult for sunlight to reach the surface of the planet. Therefore (and also as a result of the large number of tiny particles of volcanic ash in the upper atmosphere, which also impede sunlight access to the planet), significant climate changes can occur after particularly strong volcanic eruptions. For example, as a result of the eruption of the Ksudach volcano (Kamchatka Peninsula, 1907), an increased concentration of dust in the atmosphere remained for about 2 years, and characteristic noctilucent clouds of sulfuric acid were observed even in Paris. The explosion of Mount Pinatubo in 1991, which released 3 × 10 7 tons of sulfur into the atmosphere, resulted in 1992 and 1993 being significantly colder than 1991 and 1994.
Standards
- Technical sulfuric acid GOST 2184-77
- Battery sulfuric acid. Technical specifications GOST 667-73
- Sulfuric acid of special purity. Technical specifications GOST 1422-78
- Reagents. Sulfuric acid. Technical specifications GOST 4204-77
Any acid is a complex substance whose molecule contains one or more hydrogen atoms and an acid residue.
The formula of sulfuric acid is H2SO4. Consequently, the sulfuric acid molecule contains two hydrogen atoms and the acidic residue SO4.
Sulfuric acid is formed when sulfur oxide reacts with water
SO3+H2O -> H2SO4
Pure 100% sulfuric acid (monohydrate) is a heavy liquid, viscous like oil, colorless and odorless, with a sour “copper” taste. Already at a temperature of +10 ° C it hardens and turns into a crystalline mass.
Concentrated sulfuric acid contains approximately 95% H2SO4. And it hardens at temperatures below –20°C.
Interaction with water

Sulfuric acid dissolves well in water, mixing with it in any proportion. This releases a large amount of heat.
Sulfuric acid can absorb water vapor from the air. This property is used in industry for drying gases. The gases are dried by passing them through special containers with sulfuric acid. Of course, this method can only be used for those gases that do not react with it.
It is known that when sulfuric acid comes into contact with many organic substances, especially carbohydrates, these substances become charred. The fact is that carbohydrates, like water, contain both hydrogen and oxygen. Sulfuric acid takes these elements away from them. What remains is coal.
In an aqueous solution of H2SO4, the indicators litmus and methyl orange turn red, which indicates that this solution has a sour taste.
Interaction with metals
Like any other acid, sulfuric acid is capable of replacing hydrogen atoms with metal atoms in its molecule. It interacts with almost all metals.
Diluted sulfuric acid reacts with metals like an ordinary acid. As a result of the reaction, a salt with an acidic residue SO4 and hydrogen is formed.
Zn + H2SO4 = ZnSO4 + H2
A concentrated sulfuric acid is a very strong oxidizing agent. It oxidizes all metals, regardless of their position in the voltage series. And when reacting with metals, it itself is reduced to SO2. Hydrogen is not released.
Сu + 2 H2SO4 (conc) = CuSO4 + SO2 + 2H2O
Zn + 2 H2SO4 (conc) = ZnSO4 + SO2 + 2H2O
But gold, iron, aluminum, and platinum group metals do not oxidize in sulfuric acid. Therefore, sulfuric acid is transported in steel tanks.
The sulfuric acid salts that are obtained as a result of such reactions are called sulfates. They are colorless and easily crystallize. Some of them are highly soluble in water. Only CaSO4 and PbSO4 are slightly soluble. BaSO4 is almost insoluble in water.
Interaction with bases

The reaction between acids and bases is called neutralization reaction. As a result of the neutralization reaction of sulfuric acid, a salt containing the acid residue SO4 and water H2O are formed.
Examples of sulfuric acid neutralization reactions:
H2SO4 + 2 NaOH = Na2SO4 + 2 H2O
H2SO4 + CaOH = CaSO4 + 2 H2O
Sulfuric acid reacts with neutralization with both soluble and insoluble bases.
Since the sulfuric acid molecule has two hydrogen atoms, and two bases are required to neutralize it, it is classified as a dibasic acid.
Interaction with basic oxides
From the school chemistry course we know that oxides are complex substances that contain two chemical elements, one of which is oxygen in the oxidation state -2. Basic oxides are called oxides of 1, 2 and some 3 valence metals. Examples of basic oxides: Li2O, Na2O, CuO, Ag2O, MgO, CaO, FeO, NiO.
Sulfuric acid reacts with basic oxides in a neutralization reaction. As a result of this reaction, as in the reaction with bases, salt and water are formed. The salt contains the acidic residue SO4.
CuO + H2SO4 = CuSO4 + H2O
Interaction with salts
Sulfuric acid reacts with salts of weaker or volatile acids, displacing these acids from them. As a result of this reaction, a salt with an acidic residue SO4 and an acid are formed
H2SO4+BaCl2=BaSO4+2HCl
Application of sulfuric acid and its compounds

Barium porridge BaSO4 is capable of blocking X-rays. Filling the hollow organs of the human body with it, radiologists examine them.
In medicine and construction, natural gypsum CaSO4 * 2H2O and calcium sulfate crystalline hydrate are widely used. Glauber's salt Na2SO4 * 10H2O is used in medicine and veterinary medicine, in the chemical industry - for the production of soda and glass. Copper sulfate CuSO4 * 5H2O is known to gardeners and agronomists, who use it to combat pests and plant diseases.
Sulfuric acid is widely used in various industries: chemical, metalworking, oil, textile, leather and others.