Classification and properties of complex inorganic substances. Relationship. Classification of inorganic compounds and their properties Classification of inorganic compounds oxides acids bases salts
Oxides– compounds of elements with oxygen, the oxidation state of oxygen in oxides is always -2.
Basic oxides form typical metals with C.O. +1,+2 (Li 2 O, MgO, CaO, CuO, etc.).
Acidic oxides form nonmetals with S.O. more than +2 and metals with S.O. from +5 to +7 (SO 2, SeO 2, P 2 O 5, As 2 O 3, CO 2, SiO 2, CrO 3 and Mn 2 O 7). Exception: the oxides NO 2 and ClO 2 do not have corresponding acidic hydroxides, but they are considered acidic.
Amphoteric oxides formed by amphoteric metals with C.O. +2,+3,+4 (BeO, Cr 2 O 3, ZnO, Al 2 O 3, GeO 2, SnO 2 and PbO).
Non-salt-forming oxides– non-metal oxides with CO+1,+2 (CO, NO, N 2 O, SiO).
Reasons (main hydroxides ) - complex substances that consist of a metal ion (or ammonium ion) and a hydroxyl group (-OH).
Acidic hydroxides (acids)- complex substances that consist of hydrogen atoms and an acid residue.
Amphoteric hydroxides formed by elements with amphoteric properties.
Salts- complex substances formed by metal atoms combined with acidic residues.
Medium (normal) salts- all hydrogen atoms in acid molecules are replaced by metal atoms.
Acid salts- hydrogen atoms in the acid are partially replaced by metal atoms. They are obtained by neutralizing a base with an excess of acid. To correctly name sour salt, It is necessary to add the prefix hydro- or dihydro- to the name of a normal salt, depending on the number of hydrogen atoms included in the acid salt.
For example, KHCO 3 - potassium bicarbonate, KH 2 PO 4 - potassium dihydrogen orthophosphate
It must be remembered that acid salts can only form two or more basic acids.
Basic salts- hydroxo groups of the base (OH −) are partially replaced by acidic residues. To name basic salt, it is necessary to add the prefix hydroxo- or dihydroxo- to the name of a normal salt, depending on the number of OH groups included in the salt.
For example, (CuOH) 2 CO 3 is copper (II) hydroxycarbonate.
It must be remembered that basic salts can only form bases containing two or more hydroxo groups.
Double salts- they contain two different cations; they are obtained by crystallization from a mixed solution of salts with different cations, but the same anions. For example, KAl(SO 4) 2, KNaSO 4.
Mixed salts- they contain two different anions. For example, Ca(OCl)Cl.
Hydrate salts (crystal hydrates) - they contain molecules of water of crystallization. Example: Na 2 SO 4 10H 2 O.
Trivial names of commonly used inorganic substances:
| Formula | Trivial name |
| NaCl | halite, rock salt, table salt |
| Na 2 SO 4 *10H 2 O | Glauber's salt |
| NaNO3 | Sodium, Chilean nitrate |
| NaOH | caustic soda, caustic soda, caustic soda |
| Na 2 CO 3 *10H 2 O | crystal soda |
| Na 2 CO 3 | Soda Ash |
| NaHCO3 | baking (drinking) soda |
| K2CO3 | potash |
| CON | caustic potassium |
| KCl | potassium salt, sylvite |
| KClO3 | Berthollet's salt |
| KNO 3 | Potassium, Indian saltpeter |
| K 3 | red blood salt |
| K 4 | yellow blood salt |
| KFe 3+ | Prussian blue |
| KFe 2+ | Turnbull blue |
| NH4Cl | Ammonia |
| NH 3 *H 2 O | ammonia, ammonia water |
| (NH 4) 2 Fe(SO 4) 2 | Mohr's salt |
| CaO | quicklime (burnt) lime |
| Ca(OH) 2 | slaked lime, lime water, milk of lime, lime dough |
| СaSO 4 *2H 2 O | Gypsum |
| CaCO3 | marble, limestone, chalk, calcite |
| CaHPO 4 × 2H2O | Precipitate |
| Ca(H 2 PO 4) 2 | double superphosphate |
| Ca(H 2 PO 4) 2 +2CaSO 4 | simple superphosphate |
| CaOCl 2 (Ca(OCl) 2 + CaCl 2) | bleaching powder |
| MgO | magnesia |
| MgSO 4 *7H 2 O | Epsom (bitter) salt |
| Al2O3 | corundum, bauxite, alumina, ruby, sapphire |
| C | diamond, graphite, soot, coal, coke |
| AgNO3 | lapis |
| (CuOH) 2 CO 3 | malachite |
| Cu2S | copper luster, chalcocite |
| CuSO 4 *5H 2 O | copper sulfate |
| FeSO 4 *7H 2 O | inkstone |
| FeS 2 | pyrite, iron pyrite, sulfur pyrite |
| FeCO 3 | siderite |
| Fe 2 O 3 | red iron ore, hematite |
| Fe 3 O 4 | magnetic iron ore, magnetite |
| FeO × nH 2 O | brown iron ore, limonite |
| H2SO4 × nSO 3 | oleum solution of SO 3 in H 2 SO 4 |
| N2O | laughing gas |
| NO 2 | brown gas, foxtail |
| SO 3 | sulfur gas, sulfuric anhydride |
| SO 2 | sulfur dioxide, sulfur dioxide |
| CO | carbon monoxide |
| CO2 | carbon dioxide, dry ice, carbon dioxide |
| SiO2 | silica, quartz, river sand |
| CO+H2 | water gas, synthesis gas |
| Pb(CH3COO)2 | lead sugar |
| PbS | lead luster, galena |
| ZnS | zinc blende, sphalerite |
| HgCl2 | corrosive sublimate |
| HgS | cinnabar |
The classification of inorganic substances is based on their ability to decompose. Simple substances, consisting of atoms of only one chemical element (O 2, H 2, Mg), do not disintegrate. Complex substances consisting of atoms of two or more elements (CO 2, H 2 SO 4, NaOH, KCl) easily decompose.
Simple
Classification of classes of inorganic substances includes:
- metals - elements with thermal and electrical conductivity, high ductility, malleability, and metallic luster;
- nonmetals - elements that are more fragile than metals, do not have electrical conductivity and exhibit oxidizing properties.

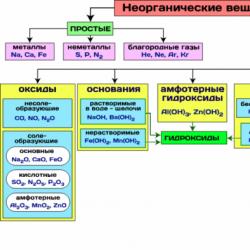
Rice. 1. Classification scheme for inorganic substances.
Metals are located in the lower left corner of the periodic table, nonmetals are located in the upper right corner and include the noble gases.

Rice. 2. The location of metals and non-metals in the periodic table.
Many simple chemical elements have allotropy - the property of forming several simple substances. For example, when one more atom is added to oxygen, the simple substance ozone (O 3) is formed; carbon, depending on the number of atoms, forms graphite, coal or diamond.
Complex
Complex substances are classified into the following classes:
- oxides - consist of two elements, one of which is oxygen;
- acids - consist of hydrogen atoms and an acid residue;
- grounds - consist of a metal and one or more hydroxyl groups;
- salt - consist of metal and acid residue.
Separately, amphoteric hydroxides are isolated, which exhibit the properties of acids and bases. These are solids that are weak electrolytes. These include metal hydroxides with oxidation states +3 and +4. Exceptions are Be(OH)2, Zn(OH)2, Sn(OH)2, Pb(OH)2.
A more detailed classification of complex substances is presented in the table with examples.
|
View |
Nomenclature |
Chemical properties |
Example |
|
Oxides - E x O y |
Element oxide (oxidation state) |
There are basic oxides, which form salts when interacting with acids, and acidic oxides, which form acids when interacting with bases. Separately, amphoteric oxides are isolated that interact with acids and bases (salt is formed) |
Na 2 O - sodium oxide, Fe 2 O 3 - iron (III) oxide, N 2 O 5 - nitric oxide (V) |
|
Bases - Me(OH) x |
Metal hydroxide (oxidation state) |
In accordance with solubility, alkalis and water-insoluble bases are distinguished. Alkalis react with non-metals and acid oxides. Insoluble bases react with acids and can decompose at high temperatures |
Fe(OH) 2 - iron (II) hydroxide, Cu(OH) 2 - copper (II) hydroxide, NaOH - sodium hydroxide |
|
Acids - H n Ac |
Read depending on acid residue |
They interact with metals to the left of hydrogen in the activity series, with oxides and salts. Capable of decomposition at high temperatures |
H 2 SO 4 - sulfuric acid, HCl - hydrochloric acid, HNO 3 - nitric acid |
|
Salts - Fur x (Ac) y |
Acid residue of metal (oxidation state) |
Reacts with acids, alkalis, metals and salts |
Na 2 SO 4 - sodium sulfate, CaCO 3 - calcium carbonate, KCl - potassium chloride |

Rice. 3. List of names of acids.
Genetic connections between classes are based on the mutual transformation of substances. During chemical reactions, atoms move from one substance to another, forming genetic series (series of transformations). When a metal is added to oxygen, it forms an oxide, which when reacted with water turns into a base. An acid oxide is formed from a non-metal, which reacts with water to form an acid. Any genetic series ends with salt.
What have we learned?
Inorganic substances include simple and complex compounds. Simple substances are made up of atoms of the same element. These include metals and non-metals. Complex compounds include substances consisting of several elements. These include oxides, acids, bases, salts and amphoteric hydroxides. All substances are genetically related to each other. From a simple substance you can get a more complex substance. Salts are considered the most complex substances.
Test on the topic
Evaluation of the report
Average rating: 4.6. Total ratings received: 102.
Simple substances. Molecules are made up of atoms of the same type (atoms of the same element). In chemical reactions they cannot decompose to form other substances.
Complex substances (or chemical compounds). Molecules consist of different types of atoms (atoms of different chemical elements). In chemical reactions they decompose to form several other substances.
There is no sharp boundary between metals and non-metals, because There are simple substances that exhibit dual properties.
Allotropy
Allotropy- the ability of some chemical elements to form several simple substances that differ in structure and properties.
C - diamond, graphite, carbine.
O - oxygen, ozone.
S - rhombic, monoclinic, plastic.
P - white, red, black.
The phenomenon of allotropy is caused by two reasons:
1) different numbers of atoms in the molecule, for example oxygen O 2 and ozone O 3
2) the formation of various crystalline forms, for example diamond and graphite.
BASES
Reasons- complex substances in which metal atoms are connected to one or more hydroxyl groups (from the point of view of the theory of electrolytic dissociation, bases are complex substances, upon dissociation of which in an aqueous solution, metal cations (or NH 4 +) and hydroxide - anions OH - are formed) .
Classification. Soluble in water (alkalies) and insoluble. Amphoteric bases also exhibit the properties of weak acids.
Receipt
1. Reactions of active metals (alkali and alkaline earth metals) with water:
2Na + 2H 2 O ® 2NaOH + H 2 -
Ca + 2H 2 O ® Ca(OH) 2 + H 2 -
2. Interaction of active metal oxides with water:
BaO + H 2 O ® Ba(OH) 2
3. Electrolysis of aqueous salt solutions
2NaCl + 2H 2 O ® 2NaOH + H 2 - + Cl 2 -
Chemical properties
| Alkalis | Insoluble bases |
| 1. Action on indicators. | |
| litmus - blue methyl orange - yellow phenolphthalein - raspberry |
-- |
| 2. Interaction with acid oxides. | |
| 2KOH + CO 2 ® K 2 CO 3 + H 2 O KOH + CO 2 ® KHCO 3 |
-- |
| 3. Interaction with acids (neutralization reaction) | |
| NaOH + HNO 3 ® NaNO 3 + H 2 O | Cu(OH) 2 + 2HCl ® CuCl 2 + 2H 2 O |
| 4. Exchange reaction with salts | |
| Ba(OH) 2 + K 2 SO 4 ® 2KOH + BaSO 4 ¯ 3KOH+Fe(NO 3) 3 ® Fe(OH) 3 ¯ + 3KNO 3 |
-- |
| 5. Thermal decomposition. | |
| -- | Cu(OH) 2 - t ° ® CuO + H 2 O |
OXIDES
Classification
Oxides- these are complex substances consisting of two elements, one of which is oxygen.
| OXIDES | |
| Non-salt-forming | CO, N2O, NO |
| Salt-forming | Basic - these are metal oxides in which the latter exhibit a small oxidation state +1, +2 Na 2 O; MgO; CuO |
| |
Amphoteric (usually for metals with oxidation state +3, +4). Amphoteric hydroxides correspond to them as hydrates ZnO; Al 2 O 3; Cr 2 O 3; SnO2 |
| |
Acidic - these are oxides of non-metals and metals with an oxidation state from +5 to +7 SO2; SO 3; P2O5; Mn 2 O 7; CrO3 |
| |
Basic oxides bases correspond acidic- acids, amphoteric- and those and others |
Receipt
1. Interaction of simple and complex substances with oxygen:
2Mg + O 2 ® 2MgO
4P + 5O 2 ® 2P 2 O 5
S + O 2 ® SO 2
2CO + O 2 ® 2CO 2
2CuS + 3O 2 ® 2CuO + 2SO 2
CH 4 + 2O 2 ® CO 2 + 2H 2 O
4NH 3 + 5O 2 - cat. ® 4NO + 6H 2 O
2. Decomposition of some oxygen-containing substances (bases, acids, salts) when heated:
Cu(OH) 2 - t ° ® CuO + H 2 O
(CuOH) 2 CO 3 - t ° ® 2CuO + CO 2 + H 2 O
2Pb(NO 3) 2 - t ° ® 2PbO + 4NO 2 + O 2
2HMnO 4 - t °;H 2 SO 4 (conc.) ® Mn 2 O 7 + H 2 O
Chemical properties
| Basic oxides | Acidic oxides |
| 1. Interaction with water | |
| The base is formed: Na 2 O + H 2 O ® 2NaOH CaO + H 2 O ® Ca(OH) 2 |
Acid is formed: SO 3 + H 2 O ® H 2 SO 4 P 2 O 5 + 3H 2 O ® 2H 3 PO 4 |
| 2. Interaction with acid or base: | |
| When reacting with acid salt and water are formed MgO + H 2 SO 4 - t ° ® MgSO 4 + H 2 O CuO + 2HCl - t ° ® CuCl 2 + H 2 O |
When reacting with a base salt and water are formed CO 2 + Ba(OH) 2 ® BaCO 3 + H 2 O SO 2 + 2NaOH ® Na 2 SO 3 + H 2 O |
| Amphoteric oxides interact | |
| with acids as bases: ZnO + H 2 SO 4 ® ZnSO 4 + H 2 O |
with bases as acidic: ZnO + 2NaOH ® Na 2 ZnO 2 + H 2 O (ZnO + 2NaOH + H 2 O ® Na 2) |
| 3. The interaction of basic and acidic oxides with each other leads to salts. | |
| Na 2 O + CO 2 ® Na 2 CO 3 | |
| 4. Reduction to simple substances: | |
| 3CuO + 2NH 3 ® 3Cu + N 2 + 3H 2 O P 2 O 5 + 5C ® 2P + 5CO |
|
And their derivatives. All other substances are inorganic.
Classification of inorganic substances
Inorganic substances are divided into simple and complex according to their composition.
Simple substances consist of atoms of one chemical element and are divided into metals, nonmetals, and noble gases. Complex substances consist of atoms of different elements chemically bonded to each other.
Complex inorganic substances, according to their composition and properties, are divided into the following important classes: oxides, bases, acids, amphoteric hydroxides, salts.
Lesson content lesson notes supporting frame lesson presentation acceleration methods interactive technologies Practice tasks and exercises self-test workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Illustrations audio, video clips and multimedia photographs, pictures, graphics, tables, diagrams, humor, anecdotes, jokes, comics, parables, sayings, crosswords, quotes Add-ons abstracts articles tricks for the curious cribs textbooks basic and additional dictionary of terms other Improving textbooks and lessonscorrecting errors in the textbook updating a fragment in a textbook, elements of innovation in the lesson, replacing outdated knowledge with new ones Only for teachers perfect lessons calendar plan for the year; methodological recommendations; discussion program Integrated LessonsClassification of substances
All substances are divided into simple (elementary) and complex. Simple substances consist of one element, complex substances - of two or more elements. Simple substances are divided into metals and non-metals.
Metals have a characteristic “metallic” luster, are malleable, malleable, can be rolled into sheets or drawn into wire, and have good thermal and electrical conductivity. At room temperature, all metals (except mercury) are in a solid state.
Nonmetals do not have the luster characteristic of metals, are brittle, and conduct heat and electricity very poorly. Some of them are gaseous under normal conditions.
Complex substances are divided into organic and inorganic (mineral). Organic compounds are usually called carbon compounds, with the exception of the simplest carbon compounds (CO, CO 2, H 2 CO 3, HCN and their salts, etc.); all other substances are called inorganic.
Complex inorganic compounds are classified both by composition and by chemical properties (functional characteristics). According to their composition, they are primarily divided into two-element, or binary, compounds (oxides, sulfides, halides, nitrides, carbides, hydrides) and multi-element compounds; oxygen-containing, nitrogen-containing, etc.
Based on their chemical properties, inorganic compounds are divided into four main classes: oxides, acids, bases, and salts.
Oxides
Oxides are complex substances consisting of two elements, one of which is oxygen(Cr 2 O 3, K 2 O, CO 2, etc.). Oxygen in oxides is always divalent and has an oxidation state of -2.
Based on their chemical properties, oxides are divided into salt-forming and non-salt-forming.(indifferent: CO, NO, N 2 O). Salt-forming oxides are divided into basic, acidic and amphoteric.
Basic are oxides that react with acids or acidic oxides to form salts:
CuO + 2HCl=CuCl 2 + H 2 O,
MgO + CO 2 = MgCO 3.
The formation of basic oxides is typical for metals with a low oxidation state (+1, +2).
Oxides of alkali (Li, Na, K, Rb, Cs) and alkaline earth metals (Ca, Sr, Ba, Ra) react with water, forming bases. For example:
Na 2 O + H 2 O = 2NaOH,
CaO + H 2 O = Ca(OH) 2.
Most basic oxides do not interact with water. The bases of such oxides are obtained indirectly:
a) CuO + 2HCl=CuCl 2 + H 2 O;
b) CuCl 2 + 2KOH = Cu(OH) 2 + 2KCl.
Oxides that react with bases or basic oxides to form salts are called acidic. For example:
SO 3 + 2KOH = K 2 SO 4 + H 2 O,
CaO + CO 2 = CaCO 3.
Acidic oxides include oxides of typical nonmetals-SO 2, N 2 O 5, SiO 2, CO 2, etc., as well as metal oxides with a high oxidation state (+5, +6, +7, +8)-V 2 O 5, CrO 3, Mn 2 O 7, etc.
A number of acidic oxides (SO 3, SO 2, N 2 O 3, N 2 O 5, CO 2, etc.) when interacting with water form acids:
SO 3 + H 2 O = H 2 SO 4,
N 2 O 5 + H 2 O = 2HNO 3.
The corresponding acids of other acidic oxides (SiO 2, TeO 2, TeO 3, MoO 3, WO 3, etc.) are obtained indirectly. For example:
a) SiO 2 + 2NaOH = Na 2 SiO 3 + H 2 O
b) Na 2 SiO 3 + 2HCl = H 2 SiO 3 + 2NaCl
One way to obtain acid oxides is to remove water from the corresponding acids. Therefore, acid oxides are sometimes called acid anhydrides.
Amphoteric are oxides that form salts when interacting with both acids and bases, i.e., having dual properties - the properties of basic and acidic oxides. For example:
SnO + H 2 SO 4 = SnSO 4 + H 2 O,
SnO + 2KOH + H 2 O = K 2,
ZnO + 2KOH = K 2 ZnO 2 + H 2 O.
Amphoteric oxides include: ZnO, BeO, SnO, PbO, Al 2 O 3, Cr 2 O 3, Fe 2 O 3, Sb 2 O 3, MnO 2 and etc.
It should be noted that in accordance with the change in the chemical nature of the elements in the periodic table of elements (from metals to non-metals), the chemical properties of compounds naturally change, in particular, the acid-base activity of their oxides. Thus, in the case of higher oxides of elements of the 3rd period in the series: Na 2 O, MgO, Al 2 O 3, SiO 2, P 2 O 5, SO 3, Cl 2 O 7 - as the degree of polarity of the E-O bond decreases (decreases DEO; the negative effective charge of the oxygen atom decreases) the basic oxides weaken and the acidic properties increase: Na 2 O, MgO - basic oxides; Al 2 O 3 – amphoteric; SiO 2 , P 2 O 5 , SO 3 , Cl 2 O 7 are acidic oxides (from left to right, the acidic nature of the oxides increases).
Methods for obtaining oxides:
1. Interaction of simple substances with oxygen (oxidation):
4Fe + 3O 2 = 2Fe 2 O 3,
S + O 2 = SO 2.
2. Combustion of complex substances:
CH 4 + 2O 2 = CO 2 + 2H 2 O,
2SO 2 + O 2 = 2SO 3.
3. Thermal decomposition of salts, bases, acids:
CaCO 3 ® CaO + CO 2,
Cd(OH) 2 ® CdO + H 2 O,
H 2 SO 4 ® SO 3 + H 2 O.
Nomenclature of oxides. The names of oxides are constructed from the word “oxide” and the name of the element in the genitive case that is connected to oxygen atoms. If an element forms several oxides, then its oxidation state (s.o.) is indicated in parentheses in Roman numerals, with the sign c. O. not specified. For example, MnO 2 is manganese (IV) oxide, MnO is manganese (II) oxide. If an element forms one oxide, then its s. O. not given: Na 2 O – sodium oxide.
Sometimes the names of oxides contain prefixes di-, tri-, tetra-, etc. They indicate that in the molecule of this oxide there are 2,3,4, etc. per atom of the element. oxygen atom, for example, CO 2 - carbon dioxide, etc.
Hydroxides
Among multielement compounds, an important group consists of hydroxides are complex substances containing OH hydroxyl groups. Some of them (basic hydroxides) exhibit the properties of bases - NaOH, Ba(OH) 2, etc.; others (acid hydroxides) exhibit the properties of acids - HNO 3, H 3 PO 4, etc.; There are also amphoteric hydroxides that, depending on conditions, can exhibit both basic and acidic properties - Zn(OH) 2, Al(OH) 3, etc.
The properties and character of hydroxides also depend on the charge of the nucleus of the central atom (symbol E) and its radius, i.e. on the strength and polarity of the E–O and O–N bonds.
If the binding energy is E O - H<< E Э - О, то диссоциация гидроксида протекает по кислотному типу, т. е. разрушается связь О – Н.
EON Û EO - + H +
If E O-H >> E E – O, then the dissociation of the hydroxide proceeds according to the main type, i.e. the E – O bond is destroyed
EOH Û E + + OH -
If the energies of the O – H and E – O bonds are close or equal, then the dissociation of the hydroxide can occur simultaneously in both directions. In this case we are talking about amphoteric hydroxides:
E n+ + nOH - Û E(OH) n = H n EO n Û nH + + EO n n-
In accordance with the change in the chemical nature of the elements in the periodic table of elements, the acid-base activity of their hydroxides naturally changes: from basic hydroxides through amphoteric to acidic. For example, for higher hydroxides of elements there are 3 periods:
NaOH, Mg(OH) 2 – bases (from left to right, the main properties weaken);
Al(OH) 3 – amphoteric hydroxide;
H 2 SiO 3 , H 3 PO 4 , H 2 SO 4 , HСlO 4 – acids (from left to right, the strength of acids increases).
Metal hydroxides are classified as bases. The more clearly the metallic properties of an element are expressed, the more pronounced are the basic properties of the corresponding metal hydroxide in the highest degree. Non-metal hydroxides exhibit acidic properties. The more pronounced the non-metallic properties of an element, the stronger the acidic properties of the corresponding hydroxide.
Acids
Acids are substances that dissociate in solutions to form hydrogen cations and anions of the acid residue (from the standpoint of the theory of electrolytic dissociation).
Acids are classified according to their strength (by their ability to electrolytically dissociate - into strong and weak), by basicity (by the number of hydrogen atoms in an acid molecule that can be replaced by metal atoms to form a salt - into monobasic, dibasic, tribasic), by the presence or absence of oxygen in the composition of acid (oxygen-containing and oxygen-free). For example, nitric acid HNO 3 is a strong, monobasic, oxygen-containing acid; Hydrogen sulfide acid H 2 S is a weak, dibasic, oxygen-free acid.
Chemical properties of acids:
1. Interaction with bases to form salt and water (neutralization reaction):
H 2 SO 4 + Cu (OH) 2 = CuSO 4 + 2H 2 O.
2. Interaction with basic and amphoteric oxides with the formation of salts and water:
2HNO 3 + MgO = Mg(NO 3) 2 + H 2 O,
H 2 SO 4 + ZnO = ZnSO 4 + H 2 O.
3. Interaction with metals. Metals that are in the “Stress Series” before hydrogen displace hydrogen from acid solutions (except for nitric and concentrated sulfuric acids); this produces salt:
Zn + 2HCl = ZnCl 2 + H 2.
Metals located in the “Stress Series” after hydrogen do not displace hydrogen from acid solutions
For the interaction of metals with nitric and concentrated sulfuric acids, see section 11.
4. Some acids decompose when heated:
H 2 SiO 3 H 2 O + SiO 2 .
5. Less volatile acids displace more volatile acids from their salts:
H 2 SO 4 conc + NaCl tv = NaHSO 4 + HCl.
6. Stronger acids displace less strong acids from solutions of their salts:
2HCl + Na 2 CO 3 = 2NaCl + H 2 O + CO 2
Nomenclature of acids. The names of oxygen-free acids are composed by adding the suffix - to the root of the Russian name of the acid-forming element (or to the name of a group of atoms, for example, CN - cyan, CNS - rhodane). O-, ending hydrogen and the word “acid”. For example, HCl is hydrochloric acid, H 2 S is hydrosulfide acid, HCN is hydrocyanic acid.
The names of oxygen-containing acids are also formed from the Russian name of the acid-forming element with the addition of appropriate suffixes, endings and the word “acid”. In this case, the name of the acid in which the element is in the highest oxidation state ends in - Naya or - new; for example, H 2 SO 4 is sulfuric acid, HClO 4 is perchloric acid, H 3 AsO 4 is arsenic acid. With a decrease in the oxidation state of the acid-forming element, the endings change in the following sequence: - oval(HClO 3 - perchloric acid), exhausted(HClO 2 - chlorous acid), - oval(HClO - hypochlorous acid). If an element forms acids while being in only two oxidation states, then the name of the acid corresponding to the lower oxidation state of the element has the ending exhausted(HNO 3 – nitric acid, HNO 2 – nitrous acid).
In some cases, a different number of water molecules can join one oxide molecule (i.e., an element in the same oxidation state forms several acids containing one atom of a given element). Then an acid with a high water content is denoted by the prefix ortho- , and an acid with a smaller number of water molecules is denoted by the prefix meta- . For example:
P 2 O 5 + H 2 O = 2HPO 3 - metaphosphoric acid;
P 2 O 5 + 3H 2 O = 2H 3 PO 4 - orthophosphoric acid.
Reasons
The basis from the standpoint of the theory of electrolytic dissociation are substances that dissociate in solutions with the formation of hydroxide ions OH ‾ and metal ions (with the exception of NH 4 OH).
Bases are classified according to their strength(according to the ability for electrolytic dissociation - into strong and weak), by acidity(by the number of hydroxo groups in the molecule that can be replaced by acid residues - monoacid, diacid, etc.), by solubility(for soluble bases - alkalis and insoluble). For example: NaOH – strong, one-acid base, soluble (alkali); Cu(OH) 2 is a weak, diacid, insoluble base. Soluble bases (alkalis) include hydroxides of alkali and alkaline earth metals. All alkalis are strong bases.
Chemical properties of bases:
1. Interaction with acids:
Ca(OH) 2 + H 2 SO 4 = CaSO 4 ¯ + H 2 O.
2. Interaction with acid oxides:
3. Interaction with amphoteric oxides:
2KOH + Al 2 O 3 = 2KAlO 2 + H 2 O 1,
2KOH + SnO + H 2 O = K 2 [Sn(OH) 4 ].
4. Interaction with amphoteric bases:
2NaOH + Zn(OH) 2 = Na 2 ZnO 2 + 2H 2 O2,
2NaOH + Zn(OH) 2 = Na 2 [Zn(OH) 4 ]3.
5. Thermal decomposition of bases with the formation of oxides and water:
Ca(OH) 2 = CaO + H 2 O.
Alkali metal hydroxides do not decompose when heated.
6. Interaction with amphoteric metals (Zn, Al, Pb, Sn, Be):
Zn + 2NaOH + 2H 2 O = Na 2 + H 2
Amphoteric hydroxides. Amphoteric hydroxides (hydrates of amphoteric oxides) are capable of dissociating in aqueous solutions both as acids and as bases. For example:
ZnO 2 2- + 2H + Û Zn(OH) 2 Û Zn 2+ + 2OH.
Therefore, they have amphoteric properties, i.e. can interact with both acids and bases:
Zn(OH) 2 + 2HCl = ZnCl 2 + 2H 2 O,
Sn(OH) 2 + 2NaOH = Na 2 [Sn(OH) 4 ].
Nomenclature of grounds. The names of the bases are built from the word “ hydroxide” and the name of the metal in the genitive case, indicating its oxidation state in parentheses in Roman numerals, if this is a variable value. Sometimes a prefix from the Greek numeral is added to the word hydroxide, indicating the number of hydroxyl groups in the base molecule. For example: KOH - potassium hydroxide; Al(OH) 3 - aluminum hydroxide (aluminum trihydroxide); Cr(OH) 2 – chromium (II) hydroxide (chromium dihydroxide).
Salts
From the point of view of the theory of electrolytic dissociation salts are substances that dissociate in solutions or melts to form positively charged ions other than hydrogen ions and negatively charged ions other than hydroxide ions.
Salts are usually considered as products of complete or partial replacement of hydrogen atoms in an acid molecule by metal atoms or products of complete or partial replacement of hydroxyl groups in a base molecule by acidic residues. With complete substitution, medium (or normal) salts are obtained that dissociate in solutions or melts to form metal cations and anions of acidic residues (with the exception of ammonium salts). When the hydrogen of an acid is incompletely replaced, acid salts are obtained; when the hydroxyl groups of a base are incompletely replaced, basic salts are obtained. The dissociation of acidic and basic salts is discussed in Section 8. Acid salts can only be formed by polybasic acids (H 2 SO 4, H 2 SO 3, H 2 S, H 3 PO 4, etc.), and basic salts can be formed by polyacid bases (Mg (OH) 2, Ca (OH) 2, Al (OH) 3, etc.).
Examples of salt formation:
Ca (OH) 2 + H 2 SO 4 = CaSO 4 + 2H 2 O,
CaSO 4 (calcium sulfate) – normal (medium) salt;
H 2 SO 4 + NaOH = NaHSO 4 + H 2 O,
NaHSO 4 (sodium hydrogen sulfate) is an acidic salt obtained as a result of a lack of a given base;
Cu (OH) 2 + HCl = CuOHCl + H 2 O,
CuOHCl (hydroxycopper(II) chloride) is the basic salt obtained as a result of a lack of acid taken.
Chemical properties of salts:
I. Salts enter into ion exchange reactions if a precipitate, a weak electrolyte is formed, or a gas is released:
Salts whose metal cations correspond to insoluble bases react with alkalis:
CuSO 4 + 2NaOH = Na 2 SO 4 + Cu (OH) 2 ↓;
Salts interact with acids:
a) the cations of which form an insoluble salt with the anion of the new acid:
BaCl 2 + H 2 SO 4 = BaSO 4 ↓ + 2HCl;
b) the anions of which correspond to an unstable carbonic or any volatile acid (in the latter case, the reaction is carried out between a solid salt and a concentrated acid):
Na 2 CO 3 + 2HCl = 2NaCl + H 2 O + CO 2,
NaCl solids + H 2 SO 4conc = NaHSO 4 + HCl;
c) the anions of which correspond to a slightly soluble acid:
Na 2 SiO 3 + 2HCl = H 2 SiO 3 ↓ + 2NaCl;
d) the anions of which correspond to a weak acid:
2CH 3 COONa + H 2 SO 4 = Na 2 SO 4 + 2CH 3 COOH;
salts interact with each other if one of the new salts formed is insoluble or decomposes (completely hydrolyzes) with the release of gas or sediment:
AgNO 3 + NaCl = NaNO 3 + AgCl↓,
2AlCl 3 + 3Na 2 CO 3 + 3H 2 O = 2Al (OH) 3 ↓ + 6NaCl + 3CO 2.
II. Salts can interact with metals if the metal to which the salt cation corresponds is located in the “Voltage Series” to the right of the reacting free metal (the more active metal displaces the less active metal from the solution of its salt):
Zn + CuSO 4 = ZnSO 4 + Cu.
III. Some salts decompose when heated:
CaCO 3 = CaO + CO 2.
IV. Some salts can react with water and form crystalline hydrates:
CuSO 4 + 5H 2 O = CuSO 4 ٭ 5H 2 O ΔH<0
white blue-blue
The release of heat and color changes are signs of chemical reactions.
V. Salts undergo hydrolysis. This process will be described in detail in section 8.10.
VI. The chemical properties of acidic and basic salts differ from the properties of average salts in that acidic salts also enter into all reactions characteristic of acids, and basic salts enter into all reactions characteristic of bases. For example:
NaHSO 4 + NaOH = Na 2 SO 4 + H 2 O,
MgOHCl + HCl = MgCl 2 + H 2 O.
Obtaining salts:
1. Interaction of the main oxide with an acid:
CuO + H 2 SO 4 = CuSO 4 + H 2 O.
2. Interaction of a metal with a salt of another metal:
Mg + ZnCl 2 = MgCl 2 + Zn.
3. Interaction of metal with acid:
Mg + 2HCl = MgCl 2 + H 2.
4. Interaction of a base with an acid oxide:
Ca(OH) 2 + CO 2 = CaCO 3 + H 2 O.
5. Interaction of base with acid:
Fe(OH) 3 + 3HCl= FeCl 3 + 3H 2 O.
6. Interaction of salt with base:
FeCl 2 + 2KOH = Fe(OH) 2 ¯ + 2KCl.
7. Interaction of two salts:
Ba(NO 3) 2 + K 2 SO 4 = BaSO 4 ¯ + 2KNO 3.
8. Interaction of metal with non-metal:
9. Interaction of acid with salt:
CaCO 3 + 2HCl = CaCl 2 + H 2 O + CO 2.
10. Interaction between acidic and basic oxides:
CaO + CO 2 = CaCO 3.
Nomenclature of salts. According to international nomenclature rules, the names of medium salts are formed from the name of the acid residue in the nominative case and the name of the metal in the genitive case, indicating its oxidation degree in parentheses in Roman numerals (if this is a variable value). The name of the acid residue consists of the root of the Latin name of the acid-forming element, the corresponding ending and, in some cases, a prefix.
Acidic residues of oxygen-free acids receive the ending eid. For example: SnS – tin (II) sulfide, Na 2 Se – sodium selenide. The endings of the names of acidic residues of oxygen-containing acids depend on the degree of oxidation of the acid-forming element. For its highest oxidation state (“-th” or “-ic” acid), the ending is used -at. For example, salts of nitric acid HNO 3 are called nitrates, sulfuric acid H 2 SO 4 - sulfates, chromic acid H 2 CrO 4 - chromates. For a lower degree of oxidation of the acid-forming element (“...pure acid”), the ending is used it. Thus, salts of nitrous acid HNO2 are called nitrites, and salts of sulfurous acid H2SO3 are called sulfites. If there is an acid with an even lower oxidation state of the acid-forming element (“-ovous acid”), its anion receives the prefix hypo- and the ending - it. For example, salts of hypochlorous acid HClO are called hypochlorites.
Salts of some acids, in accordance with historical tradition, have retained names that differ from the systematic ones. Thus, salts of manganese acid HMnO 4 are called permanganates, perchloric acid HClO 4 - perchlorates, and periodic acid HIO 4 - periodates. Salts of manganous acid H 2 MnO 4 , chloric acid HClO 3 and iodic acid HIO 3 are called manganates, chlorates and iodates, respectively.
The names of acidic and basic salts are formed according to the same general rules as the names of intermediate salts. In this case, the name of the acid salt anion is provided with the prefix hydro-, indicating the presence of unsubstituted hydrogen atoms; The number of unsubstituted hydrogen atoms is indicated by Greek numeral prefixes. For example, Na 2 HPO 4 is sodium hydrogen orthophosphate, NaH 2 PO 4 is sodium dihydrogen orthophosphate.
Similarly, the base salt cation receives the prefix hydroxo-, indicating the presence of unsubstituted hydroxo groups. The number of hydroxyl groups is indicated by Greek numerals. For example, Cr(OH) 2 NO 3 is dihydroxochrome (III) nitrate.
The names of the most important acids and their acid residues are given in Table. 4.1.
Table 4.1
Names and formulas of acids and their acid residues
Continuation of the table. 4.1