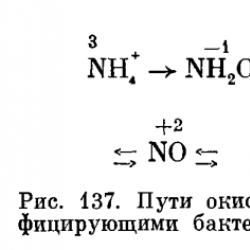Groups of nitrifiers. The meaning of nitrifying bacteria in the modern explanatory dictionary, BSE Nitrifying bacteria in agriculture
Ammonia, formed in soil, manure and water during the decomposition of organic matter, is quickly oxidized to nitrous and then nitric acid. This process is called nitrification.
Until the middle of the 19th century, more precisely, before the work of L. Pasteur, the phenomenon of nitrate formation was explained as a chemical reaction of ammonia oxidation by atmospheric oxygen, and it was assumed that the soil played the role of a chemical catalyst. L. Pasteur suggested that the formation of nitrates is a microbiological process. The first experimental evidence of this assumption was obtained by T. Schlesing and A. Münz in 1879. These researchers passed wastewater through a long column of sand and CaCO3. During filtration, ammonia gradually disappeared and nitrates appeared. Heating the column or adding antiseptics stopped the oxidation of ammonia.
However, neither the mentioned researchers nor the microbiologists who continued to study nitrification were able to isolate cultures of nitrification pathogens. Only in 1890-1892. S. N. Vinogradsky, using a special technique, isolated pure cultures of nitrifiers. S. N. Vinogradsky made the assumption that nitrifying bacteria do not grow on ordinary nutrient media containing organic substances. This was quite correct and explained the failures of his predecessors. Nitrifiers turned out to be chemolithoautotrophs, very sensitive to the presence of organic compounds in the environment. These microorganisms were isolated using mineral nutrient media.
S. N. Vinogradsky established that there are two groups of nitrifiers - one group oxidizes ammonia to nitrous acid (NH4+→NO2-) - the first phase of nitrification, the other oxidizes nitrous acid to nitric acid (NO2-→NO3-) - the second phase of nitrification.
Bacteria of both groups are currently classified in the family Nitrobacteriaceae. These are single-celled gram-negative bacteria. Among nitrifying bacteria there are species with very different morphologies - rod-shaped, ellipsoidal, spherical, convoluted and lobed, pleomorphic. The cell sizes of different species of Nitrobacteriaceae range from 0.3 to 1 µm in width and from 1 to 6.5 µm in length. There are mobile and immobile forms with polar, subpolar and peritrichial flagellation. They reproduce mainly by division, with the exception of Nitrobacter, which reproduces by budding. Almost all nitrifiers have a well-developed system of intracytoplasmic membranes, which vary significantly in shape and location in the cells of different species. These membranes are similar to those of photosynthetic purple bacteria.
Bacteria of the first phase of nitrification are represented by five genera: Nitrosomonas, Nitrosococcus, Nitrosospira, Nitrosolobus and Nitrosovibrio. The only microorganism that has been studied in detail to date is Nitrosomonas europaea.
Nitrosomonas are short oval rods measuring 0.8 - 1X1-2 microns. In liquid culture, Nitrosomonas undergo a number of developmental stages. The two main ones are represented by a mobile form and immobile zooglea. The motile form has a subpolar flagellum or a bundle of flagella. In addition to Nitrosomonas, representatives of other bacterial genera have been described that cause the first phase of nitrification.
The second phase of nitrification is carried out by representatives of the genera Nitrobacter, Nitrospira and Nitrococcus. The largest number of studies have been carried out with Nitrobacter winogradskii, but other species have also been described (Nitrobacter agilis, etc.).
Nitrobacter are elongated, wedge- or pear-shaped, with the narrower end often curved into a beak-like shape. According to the research of G. A. Zavarzin, Nitrobacter reproduction occurs by budding, and the daughter cell is usually mobile, since it has one laterally located flagellum. The alternation of mobile and immobile stages in the development cycle is known. Other bacteria that cause the second phase of nitrification have also been described.
Nitrifying bacteria are usually cultured on simple mineral media containing ammonia or nitrites (oxidizable substrates) and carbon dioxide (the main carbon source). These organisms use ammonia, hydroxylamine and nitrites as nitrogen sources.
Nitrifying bacteria develop at pH 6-8.6, the optimum pH is 7.5-8. At a pH below 6 and above 9.2, these bacteria do not develop. The optimal temperature for the development of nitrifiers is 25-30°C. A study of the relationship of different strains of Nitrosomonas europaea to temperature showed that some of them have an optimum development at 26°C or about 40°C, while others can grow quite quickly at 4°C.
Nitrifiers are obligate aerobes. With the help of oxygen, they oxidize ammonia to nitrous acid (the first phase of nitrification):
NH4++11/22О2→NO2-+H2O+2H+
And then nitrous acid to nitric acid (second phase of nitrification):
NO2-+1/2O2→NO3-
It is believed that the nitrification process occurs in several stages. The first product of ammonia oxidation is hydroxyls, which is then converted to nitroxide (NOH), or peroxonitrite (ONOOH), which, in turn, is further converted to nitrite or nitrite and nitrate.
Nitroxyl, like hydroxylamine, can apparently dimerize to hyponitrite or convert to nitrous oxide N2O, a by-product of the nitrification process.
In addition to the first reaction (formation of hydroxylamine from ammonium), all subsequent transformations are accompanied by the synthesis of high-energy bonds in the form of ATP, necessary for microbial cells to bind CO2 and other biosynthetic processes.
CO2 fixation by nitrifiers occurs through the reductive pentose phosphate cycle, or Calvin cycle. As a result of carbon dioxide fixation, not only carbohydrates are formed, but also other compounds important for bacteria - proteins, nucleic acids, fats, etc.
According to the ideas that existed until recently, nitrifying bacteria were classified as obligate chemolithoautotrophs.
Data have now been obtained indicating the ability of nitrifying bacteria to use some organic substances. Thus, a stimulating effect on the growth of Nitrobacter was noted in the presence of nitrite from yeast autolysate, pyridoxine, glutamic acid and serine. Therefore, it is assumed that nitrifying bacteria have the ability to switch from autotrophic to heterotrophic nutrition. Nitrifying bacteria still do not grow on conventional nutrient media, since the large amount of easily digestible organic substances contained in such media retards their development.
The negative attitude of these bacteria towards organic matter in laboratory conditions would seem to contradict their natural habitat. It is known that nitrifying bacteria develop well, for example, in chernozems, manure, composts, that is, in places where there is a lot of organic matter.
However, this contradiction is easily eliminated if we compare the amount of easily oxidizable carbon in the soil with the concentrations of organic matter that nitrifiers maintain in crops. Thus, soil organic matter is represented mainly by humic substances, which, for example, account for 71-91% of total carbon in chernozem , and digestible water-soluble organic matter constitutes no more than 0.1% of total carbon. Consequently, nitrifiers do not encounter large quantities of easily digestible organic matter in the soil.
The staged nature of the nitrification process is a typical example of the so-called metabiosis, that is, this kind of trophic relationships of microbes when one microorganism develops after another on the waste of its vital activity. As has been shown, ammonia, a waste product of ammonifying bacteria, is used by Nitrosomonas, and nitrites, formed last, serve as a source of life for Nitrobacter.
The question arises about the importance of nitrification for agriculture. Nitrate accumulation occurs at different rates in different soils. However, this process is directly dependent on soil fertility. The richer the soil, the more nitric acid it can accumulate. There is a method for determining the nitrogen available to plants in the soil based on its nitrification capacity. Therefore, the intensity of nitrification can be used to characterize the agronomic properties of the soil.
At the same time, during nitrification, only the conversion of one plant nutrient - ammonia - into another form - nitric acid. Nitrates, however, have some undesirable properties. While the ammonium ion is absorbed by the soil, nitric acid salts are easily washed out of it. In addition, nitrates can be reduced to N2 by denitrification, which also depletes the soil's nitrogen reserves. All this significantly reduces the utilization rate of nitrates by plants. In a plant organism, nitric acid salts, when used for synthesis, must be reduced, which requires energy. Ammonium is used directly. In this regard, the question is raised about approaches to artificially reducing the intensity of the nitrification process by using specific inhibitors that suppress the activity of bacteria - nitrifiers and are harmless to other organisms.
It should be noted that some heterotrophic microorganisms are capable of nitrification. Heterotrophic nitrifiers include bacteria from the genera Pseudomonas, Arthrobacter, Corynebacterium, Nocardia and some fungi from the genera Fusarium, Aspergillus, Penicillium, Cladosporium. It was established that Arthrobacter sp. oxidizes ammonia in the presence of organic substrates to form hydroxylamine, and then nitrite and nitrate.
Some bacteria are capable of causing nitrification of nitrogen-containing organic substances such as amides, amines, hydroxamic acids, nitro compounds (aliphatic and aromatic), oximes, etc.
Heterotrophic nitrification occurs in natural conditions (soils, reservoirs and other substrates). It can acquire dominant importance, especially in atypical conditions (for example, with a high content of organic C - and N - compounds in alkaline soil, etc.). Heterotrophic microorganisms not only contribute to nitrogen oxidation under these atypical conditions, but can also cause the formation and accumulation of toxic substances; substances with carcinogenic and mutagenic effects, as well as compounds with chemotherapeutic effects. Because some of these compounds are harmful to humans and animals even at relatively low concentrations, their formation in natural conditions should be carefully studied.
NITRIFYING BACTERIA
convert ammonia and ammonium salts into nitric acid salts - nitrates: nitrosobacteria, nitrobacteria. Distributed in soils and water bodies.
TSB. Modern explanatory dictionary, TSB. 2003
See also interpretations, synonyms, meanings of the word and what NITRIFYING BACTERIA are in Russian in dictionaries, encyclopedias and reference books:
- NITRIFYING BACTERIA
convert ammonia and ammonium salts into nitric acid salts - nitrates: nitrosobacteria, nitrobacteria. Distributed in soils and... - NITRIFYING BACTERIA
bacteria, bacteria that convert ammonia and ammonium salts into nitrates; aerobic, gram-negative, motile (have flagella); live in soil and water bodies. ... - BACTERIA in the Encyclopedia Biology:
, microscopic, usually unicellular organisms, which are characterized by the absence of a formed nucleus (see prokaryotes). Distributed everywhere: in soil, water, air, ... - BACTERIA in the Big Encyclopedic Dictionary:
(from the Greek bakterion - stick) a group of microscopic, predominantly unicellular organisms. They belong to the “pre-nuclear” forms - prokaryotes. The basis of the modern classification... - BACTERIA in the Great Soviet Encyclopedia, TSB:
(Greek bakterion - rod), a large group (type) of microscopic, predominantly unicellular organisms with a cell wall, containing a lot of deoxyribonucleic acid (DNA), having ... - BACTERIA
- BACTERIA in the Modern Encyclopedic Dictionary:
(from the Greek bakterion - stick), a group of microscopic predominantly single-celled organisms. They have a cell wall, but do not have a clearly defined nucleus. Reproducing... - BACTERIA in the Encyclopedic Dictionary:
[from ancient Greek (pal (och) ka)] lower single-celled plant organisms, visible only under a microscope. widespread in nature (cause rotting, fermentation... - NITRIFYING
NITRIFYING BACTERIA convert ammonia and ammonium salts into nitrogen salts - nitrates: nitrosobacteria, nitrobacteria. Distributed in soils and... - BACTERIA in the Big Russian Encyclopedic Dictionary:
BACTERIA (from the Greek bakt;rion - stick), microscopic group, mainly. unicellular organisms. They belong to the “pre-nuclear” forms - prokaryotes. Depending on the … - BACTERIA
- BACTERIA in Collier's Dictionary:
a large group of unicellular microorganisms characterized by the absence of a cell nucleus surrounded by a membrane. However, the genetic material of the bacterium (deoxyribonucleic acid, or DNA) ... - BACTERIA in the New Dictionary of Foreign Words:
((gr. bakteria pal(och)ka) group (type) of microscopic, predominantly unicellular organisms that have a cell wall, but do not have a formed nucleus (its role is ... - BACTERIA in the Dictionary of Foreign Expressions:
[group (type) microscopic, predominantly. single-celled organisms that have a cell wall, but do not have a formed nucleus (its role is played by a deoxyribonucleic acid molecule... - BACTERIA in the New Explanatory Dictionary of the Russian Language by Efremova:
pl. Unicellular... - BACTERIA in Lopatin’s Dictionary of the Russian Language:
bacteria, -y, units. -`eria, ... - BACTERIA in the Complete Spelling Dictionary of the Russian Language:
bacteria, units -eria,... - BACTERIA in the Spelling Dictionary:
bacteria, -y, units. -`eria, ... - BACTERIA in the Modern Explanatory Dictionary, TSB:
(from the Greek bakterion - stick), a group of microscopic, predominantly unicellular organisms. They belong to the “pre-nuclear” forms - prokaryotes. The basis of the modern classification... - BACTERIA in Ephraim's Explanatory Dictionary:
bacteria pl. Unicellular... - BACTERIA in the New Dictionary of the Russian Language by Efremova:
pl. Unicellular... - BACTERIA in the Large Modern Explanatory Dictionary of the Russian Language:
pl. Unicellular... - BACTERIA: BACTERIA AND DISEASES in Collier's Dictionary.
- MICROORGANISM NITRIFYING in Medical terms:
(syn. nitrifying bacteria) aerobic soil bacteria that cause the oxidation of ammonia and ammonium salts into nitrites, and nitrites into nitrates with the release of ... - BACTERIA NITRIFYING in Medical terms:
see Microorganisms... - CHROMOGENIC BACTERIA
forming various dyes or pigments, as a result of which their accumulations in nature and in artificial cultures are colored in different... - SULFUR BACTERIA in the Encyclopedic Dictionary of Brockhaus and Euphron.
- GLOWING BACTERIA in the Encyclopedic Dictionary of Brockhaus and Euphron:
(photogenic) - one of the remarkable physiological groups among bacteria. They are the cause of the glow, otherwise phosphorescence, of the dead inhabitants of the seas of fish, crayfish, and... - CHROMOGENIC BACTERIA
? forming various dyes or pigments, as a result of which their accumulations in nature and in artificial cultures are colored ... - SULFUR BACTERIA* in the Encyclopedia of Brockhaus and Efron.
- GLOWING BACTERIA in the Brockhaus and Efron Encyclopedia:
(photogenic) ? one of the remarkable physiological groups among bacteria. They? the reason for the glow, otherwise phosphorescence, of the dead inhabitants of the seas... - BACTERIA: STRUCTURE AND LIFE ACTIVITY OF BACTERIA in Collier's Dictionary:
To the article BACTERIA Bacteria are much smaller than the cells of multicellular plants and animals. Their thickness is usually 0.5-2.0 microns, and their length is ... - CHEMOSYNTHETISING BACTERIA in the Encyclopedia Biology:
, use the energy of chemical reactions (oxidation of inorganic substances during respiration), as a source of carbon - carbon dioxide. Nitrifying bacteria found... - VINOGRADSKY SERGEY NIKOLAEVICH in the Brief Biographical Encyclopedia:
Vinogradsky, Sergei Nikolaevich - famous botanist, bacteriologist. Born in 1856. Educated at Kiev, St. Petersburg, Strasbourg and Zurich universities. ... - CHEMOSYNTHESIS in the Great Soviet Encyclopedia, TSB:
(from chemo... and synthesis), more correctly - chemolithoautotrophy, a type of nutrition characteristic of some bacteria capable of assimilating CO2 as the only source of carbon... - METABOLISM in the Great Soviet Encyclopedia, TSB:
substances, or metabolism, is the natural order of transformation of substances and energy in living systems underlying life, aimed at ... - MICROORGANISMS in the Great Soviet Encyclopedia, TSB:
microbes, a large group of mostly single-celled living beings, visible only under a microscope and organized more simply than plants and animals. To M.... - AEROBES in the Great Soviet Encyclopedia, TSB:
aerobic organisms (from aero... and Greek bios - life), organisms that have an aerobic type of respiration, i.e. capable of living and ...
Evgeny Granovsky
This material, conceived in ancient times, before the creation of this site, turned out to be a real trap for me. An active position on Internet forums required me to write too many words each time; I wanted to compile and systematize information so that I could simply refer to it later. In fact, this meant a new level of immersion in the dim world of pipes and sponges, instead of writing about outlandish tropical fish and distant biotopes. And, in the end, I stopped writing anything at all. The material would probably have remained on my computer if Sergei Anikshtein had not encouraged me to bring the work to some more or less readable state and, having gathered the courage, to publish it. The text, despite the fact that a number of new paragraphs have been added to it, is fairly crude. Even after years, some points seem controversial to me or, at a minimum, require additional verification. However, aquarium farming is not an area where there can and should be only one correct opinion, and sometimes opposing decisions lead to equally good results in our business. In any case, I think that the material presented will be a good starting point for the aquarist who wants to understand these issues. But the most important thing here is not to fall into excessive techno-fetishism, because there is a lot more in the world, much more interesting and entertaining than chemical reactions, filters and pumps.
* * *
In modern aquarium farming, filters are one of the most important means of life support. An aquarium is a closed biological space in which there is a constant accumulation of organic residues: fish produce secretions that pollute the water; plus uneaten food, dead parts of plants, etc. In natural reservoirs, the concentration of waste in water is quite stable, since part of it is processed into minerals and assimilated by plants, and the other part is carried out along with water flows. In an aquarium, the density of fish stocking significantly exceeds the natural one, so metabolic products and their inorganic derivatives can have a negative impact on its inhabitants.
The main ways to remove excess mineral and organic residues from an aquarium and establish acceptable living conditions for fish in it are filtration, cleaning, water changes and the use of sorbing chemistry.
Along with the skills of handling filter systems, pumps, siphons and absorbents, the aquarist must also have a certain amount of theoretical knowledge. An integral part of modern aquarium science is the so-called “nitrogen cycle”. If you open old books, you will not find a word there about biofilters or the nitrogen cycle. The first simply did not exist then, while the second proceeded on its own, which the “old school” aquarists only vaguely guessed about when speaking about a certain “biological equilibrium” that occurs by itself a few weeks after the launch. As a rule, there were densely overgrown aquariums with neutral or acidified water, inhabited by “characinka” or “livebearer”, where living plants quite energetically absorbed toxic ammonium compounds, and if they were present in small residual doses, then mainly in the form of relatively safe ammonium ions NH 4 +. Moreover, in “Dutch aquariums”, with a large number of plants and a small number of fish, nitrates were introduced artificially!
The cichlid craze that has gripped the aquarium hobby since the 1970s has required aquarists to become much more knowledgeable about biofiltration. Although before that this segment had already been largely mastered by marine aquarists. They were the first to face the problem of toxic nitrogens and began to develop appropriate water treatment systems. Following the “sailors” and cichlid keepers, other aquarists also addressed this problem, and the production of aquarium filters turned into an entire branch of the aquarium industry.
This material summarizes and systematizes personal practical experience and information gleaned from various sources. Their list is given at the end. I would like to thank everyone whose publications and statements on aqua forums helped me in compiling this material.
Nitrogen cycle, nitrification
Uneaten food and proteins excreted in excrement that are not consumed by fish are the main suppliers of organic compounds in the water, in which the cycle of biological transformations carried out by various microorganisms begins. At the first stage of this cycle, complex nitrogen-containing organic compounds are recycled into simple inorganic compounds - the so-called mineralization. Nitrogen is one of the main elements necessary for animals and plants. It is found in animal and plant proteins. As a result of the decomposition of fish excrement, food and plant residues, and dead organisms, ammonia NH 3 (ammonia) is formed. Ammonia has the ability to interact with hydrogen ions H + found in water or with water molecules, forming ammonium ions NH 4 + (ammonium):
NH 3 + H + = NH 4 +
2NH 3 + 3O 2 = 2NO 2 - + 2H + + 2H 2 O,
or 2NH 4 + + 3O 2 = 2NO 2 - + 4H + + 2H 2 O,
and then into nitrate ions (with the letter “a”) NO 3 - .
2NO 2 - + O 2 = 2NO 3 -.
The processes of oxidation of ammonia and ammonium ions to nitrite ions and then to nitrate ions are called nitrification. These processes take place in an aerobic (i.e. oxygen-rich) environment under the influence of nitrifying bacteria existing in the aquarium. The practical meaning of nitrification is to convert nitrogen compounds from very toxic forms (ammonia, nitrite) to low toxic ones (nitrate). Nitrates are also harmful, but not as much as the previous nitrogen compounds. But the nitrogen cycle does not stop there. There is also the opposite - a recovery process called denitrification, which we, for the most part, do not currently use. Therefore, in aquarium practice, the nitrogen cycle is most often considered only in terms of nitrification. In this aspect, modern aquarium keeping is actually built on the principle of “prolonged flow”, i.e. Nitrate is removed from the aquarium by replacing contaminated water with fresh water. Living plants that absorb nitrates also play a significant role. In a cichlid aquarium, where there is little or no vegetation, the biological balance of the biosystem and the health of the fish largely depend on the technical equipment and regular maintenance. Good aeration and filtration are a prerequisite for the normal functioning of such a system, and maintaining the nitrate concentration at a safe level is carried out through intensive infusions of fresh water. Despite the existence of a number of alternative solutions (including biological methods of denitrification, which will be discussed in the third part of the article), buckets and hoses are essential aquarium equipment.
Toxicity of nitrogen compounds
Nitrogen gas itself, whose molecules consist of two N 2 atoms, is chemically and biologically inert and practically harmless. But nitrogen compounds accumulating in an aquarium can harm its inhabitants. The change in the concentrations of nitrogen compounds in the aquarium during the process of nitrification is schematically shown in the graph.
|
Changing the concentrations of nitrogen compounds in the aquarium |
According to the “List of fishery standards for maximum permissible concentrations (MAC) and approximate safe exposure level (SAEL) of harmful substances for water in water bodies of fishery importance” (M.: Publishing house VNIRO, 1999), MPC of nitrogen compounds for fish:
ammonia - 0.05 mg/l;
ammonium - 0.5 mg/l;
nitrite - 0.08 mg/l (nitrite-nitrogen concentration value);
nitrate - 40 mg/l.
Although practice shows that aquarium fish can tolerate significantly higher doses of nitrogen in the short term, these values should not be exceeded.
Ammonia is a highly toxic compound. It easily enters the blood and internal organs of the fish, accumulates there and then is excreted for a very long time, up to weeks, i.e. a fish once poisoned by ammonia can die after some time, without any external signs. Ammonia poisoning also makes fish susceptible to stress and weakens their resistance to disease. The lethal level of non-ionized ammonia is approximately 0.2–0.5 mg/L for various fish species. Ammonium ions are also toxic, but to a lesser extent. Ammonia toxicity is reduced in salt water. The ratio of the concentrations of NH 3 and NH 4 + in water also depends on its acidity and temperature: in acidic and cold water there is practically no ammonia, in an alkaline and warm environment its concentration increases. Therefore, in aquarium literature it is recommended to acidify the water to prevent fish poisoning. A decrease in pH actually leads to a decrease in the toxicity of ammonia, but at the same time the activity of nitrifying bacteria that process ammonia decreases. And at a pH below 5, their vital activity practically ceases.
The problem is compounded by the fact that the tests available to us show the total concentration of ammonium compounds, without separating ammonia from ammonium. Their percentage can be determined using special tables based on pH and water temperature. But it is best to arrange filtering so that the test shows a zero value.
However, there is an opinion that at pH values of 7 or less, the risk of ammonia poisoning is practically zero. In support of this, it is indicated that in the “pre-cichlid” era, when aquarists kept mainly tropical “acid-water” fish, cases of ammonia poisoning were very rare, and this problem arose only with the advent of the “fashion” for African cichlids requiring alkaline water. In my opinion, this is an incorrect argument, because it does not take into account the essential point that in former times all aquariums, incl. nurseries, necessarily contained plants that acted as a natural biofilter and aerator, and quite successfully. Moreover, as indicated above, ammonium ions are also not harmless and, if accumulated, can cause long-term poisoning.
A separate problem is the presence of ammonium compounds (hereinafter in the text we will generally call them “ammonia”) in tap water - during the period of autumn rains and spring floods, the concentration can reach 0.5–1 mg/l. This is discussed in more detail in the article "". Moreover, here ammonia is dangerous not so much because of its absolute concentration, but because of the sharp jump in its content in the aquarium during an abundant water change.
Nitrite is also poisonous. Prolonged stay of fish in water with a nitrite-nitrogen concentration of more than 0.1 mg/l (or a total nitrite-ion concentration of more than 0.33 mg/l) is undesirable; doses of 1 mg/l can be lethal.
Note: There are two measuring scales for nitrite content: total nitrite ion concentration (NO 2 -), i.e. nitrogen and oxygen content; and nitrite-nitrogen concentration (NO 2 – N), i.e. the content of only nitrogen in the nitrite ion. The ratio of these indicators is 3.3, that is, knowing one value, you can calculate the other. Books usually indicate the nitrite-nitrogen concentration, but in aquarium tests - as a rule, the total nitrite-ion concentration.
I want to emphasize once again that in a normally functioning aquarium the ammonia and nitrite content should be zero.
Nitrates are significantly less toxic than ammonia and nitrites. The concentration of NO 3 ions is considered safe for most fish species - up to 50 mg/l. Although there are cases of aquariums with nitrate contents of up to 400 mg/l (!!!), which in no case should be considered as a recommendation for action. At the same time, there are species of cichlids, for example, among savages Uaru fernandezyepezi well-being worsens already at a concentration of 10-20 mg/l. However, even if we do not see obvious signs of poisoning or deterioration in health, the fish is apparently healthy and spawns, in the long term, nitrates are one of the main causes of hexamitosis and other diseases in problem species and older fish and have an effect even more harmful than improper feeding, although their negative effect does not appear immediately. Even in relatively small and formally “safe” concentrations, nitrates imperceptibly but surely shorten the lifespan of our pets. There is also reason to assume the presence of a toxic “cumulative effect” when combined with nitrates with a nitrite or ammonium background (with insufficient biofiltration). Therefore, for relatively unpretentious species, it is better to set a water change regime such that the NO 3 concentration is minimal. You should also avoid sudden changes in nitrate concentration, not only upward, but also downward, in particular, when transferring fish to another container or during large water changes.
Symptoms of fish poisoning with nitrogen compounds are quite well described in the literature. In particular, you can read about this in the article “Composition of aquarium water: main problems” posted on the website vitawater.ru. From there you can learn how to improve the well-being of your fish using special preparations, both branded and “folk” (salt, potassium permanganate, methylene blue). However, if you initially do everything wisely: equip the aquarium with good filtration equipment and ensure proper maintenance, i.e. eliminate the cause of the “disease”, there will be no need to deal with its symptoms.
Types of filtering
The main purpose of aquarium filters is to purify water and remove unwanted components from it (organic and mineral particles, molecules, ions, microorganisms). Filtration can be divided into three main types:
Mechanical;
- biological;
- chemical.
Mechanical filtration- trapping particles suspended in water. With mechanical filtration, the water flow passes through some finely porous material, on which relatively large particles of dirt and aquarium debris are retained. Synthetic sponges and washcloths, special foam rubber, synthetic padding polyester, etc. are usually used as a filtering substrate.
Theoretically, cleaning efficiency increases as the particle size of the filter material or the diameter of the passage channels decreases. However, this reduction is possible only to certain limits, since this begins to increase resistance to fluid flow and reduces filter performance. It is believed that it is better to use filter elements with different sizes of passage channels. Water, successively passing through layers with ever-decreasing channels, will be evenly purified throughout the entire volume of the filter. External and some types of internal filters use a multilayer filter element (ceramic rings - large-porous and fine-porous sponges - padding polyester). When a mechanical filter operates, filtered material accumulates in it, so it is necessary to regularly wash the filter element.
Mechanical filtration also includes regular cleaning of the bottom with a siphon (soil siphon) during water changes.
Biological filtration- a multi-stage, multi-stage process carried out by ammonifying and nitrifying agents to decompose organic matter and convert highly toxic ammonia, ammonium and nitrite into low-toxic nitrate (and in the full cycle - into nitrogen gas). This process can occur naturally directly in the aquarium, but to obtain a good result it requires a special device - a biofilter.
Biological and mechanical filtration are closely related. Firstly, because the same filter can serve as both mechanical and biological. Secondly, the fact that the task of cleaning an aquarium from organic impurities is solved simultaneously both biologically, through nitrification, and mechanically, that is, by directly removing dirt from the aquarium. And thus, strong mechanical filtration eases the load on the biofilter, and vice versa.
Chemical filtration, which in aquarium practice is understood primarily as sorption, is a specific species. With the help of chemical filters, harmful organic and inorganic substances are removed from aquarium (or newly filled) water, and water parameters can also be changed and beneficial substances added to it. Depending on the nature of sorption, a distinction is made between adsorbents - bodies that absorb a substance on their (usually highly developed) surface, and chemical absorbers that bind the absorbed substance, entering into a chemical interaction with it. A separate group consists of ion exchange sorbents, which absorb ions of one type from solutions and release an equivalent amount of ions of another type into the solution. Chemical filtration is the most common and popular method using activated carbon as an adsorbent. A number of other chemical fillers are also used. These are minerals from the zeolite group, synthetic ion exchange resins, and peat. Zeolites and ion exchange resins absorb ammonia, nitrates, phosphates, etc. and instead release harmless ions of sodium, chlorine, sulfate, etc. Peat slightly acidifies the water and introduces various biologically active substances into it. Chemical filtration also includes skimmer columns, which remove organic molecules from water before they are decomposed to release ammonia. Ozonizers also, to some extent, perform chemical filtration by oxidizing organic matter.
In addition to filtration, water sterilization is also used in aquarium practice as a method of purifying it. Sterilization methods are ozonation and ultraviolet irradiation.
Nitrifying bacteria
The nitrification process is an oxidation process in which oxygen naturally plays a major role. However, this process would occur much more slowly if there were not many microorganisms taking part in the aquarium. These microorganisms are also known collectively as "activated sludge".
The preliminary work is done by mineralizers that convert organic matter into ammonia. Many microscopic aquatic inhabitants have this ability. Therefore, in principle, the set of species can be different for each specific aquarium. In particular, these are bacteria Achromobacter, Micrococcus, Flavobacterium, Paracoccus etc. Their colonies are formed in stages. Some species are replacing others. Cloudiness of the water in a recently launched aquarium (the so-called “bacterial turbidity”) is precisely a manifestation of the explosive proliferation of some microorganisms, more often ciliates, with their gradual replacement by others and/or a drop in population size.
Each stage of the nitrogen cycle is carried out by its own bacteria. Ammonia-oxidizing bacteria Nitrosococcus And Nitrosomonas carry out the process NH 3 (NH 4 +) => NO 2 -, and nitrite-oxidizing Nitraspira And Nitrobacter- process NO 2 - => NO 3 - .
The process of colonizing the aquarium with beneficial bacteria occurs gradually. To work successfully, they require certain conditions: food (ammonia and nitrites), oxygen, acceptable hydrochemistry, temperature and substrate where they will settle. According to research, optimal conditions for the development of bacteria Nitraspira spp.: nitrite concentration 0.35 mM, pH 7.6–8.0, temperature 39°C. The latter, of course, does not mean the need to heat the aquarium to such an extreme temperature; for most aquarium fish it is fatal. Nitrifiers will work great at 22–28°C. It should also be remembered that at elevated temperatures and pH, the percentage of non-ionized ammonia increases.
The main residence (substrate) for ammonifying and nitrifying bacteria are filters, especially external ones, with an impressive volume and large surface area of various fillers. Of course, these bacteria also live in the water column, but there are much fewer of them there. In principle, any surface is suitable as a substrate, but it must have a sufficiently large area. The bare walls of an aquarium are not enough to create a working population. In aquariums without a biological filter, soil acts as a substrate, but there is a deficiency of another vital component of nitrification - oxygen. Therefore, a flow-through biofilter is optimal for colonizing nitrifiers. See also the article "".
Although nitrifying bacteria are present everywhere, even in chlorinated tap water and in the air (individual cells or microcolonies), in a freshly started aquarium there are catastrophically few of them. Bacteria multiply quickly - the population doubles in 12–32 hours. However, according to studies of aquariums and biofilters, nitrification takes 12 to 22 days to establish.
Initially, there are no nitrites in the aquarium, only ammonia and ammonium, and only ammonia-oxidizing bacteria are born. As nitrites appear, nitrite-oxidizing bacteria come into action. Moreover, there is reason to believe that a colony of nitrite-oxidizing bacteria is more fastidious, its growth is slower, and it can suffer more damage than the first colony under the same influence. For example, it is known that Nitraspira can be suppressed even by excess ammonia. And if you consider that their food is supplied by the first colony, it is not surprising why the delay in processing nitrites in a new aquarium can be long. And then, on the contrary, ammonia is exhausted, and ammonia-oxidizing bacteria begin to starve and reduce the population, but now there is a lot of food for nitrite-oxidizing bacteria... Thus, we get two interconnected, but unbalanced cycles with their peaks and troughs, and the task of biofiltration is to in order to synchronize and develop these cycles, and having achieved a balance of both groups of bacteria, to respond flexibly to any changes in aquarium biochemistry.
"Tipping" the biofilter
Under unfavorable conditions, for example, lack of nutrition or sufficient oxygen, bacteria turn into the so-called. inactive state (“hibernation”), when minimal energy metabolism is maintained to ensure basic cell functions. When suitable conditions return, the bacterium “wakes up.” But if the inactive period lasts too long, death and disintegration of the bacterial cell occurs.
Particularly critical for colonies of nitrifying bacteria in a biofilter are long-term power outages and severe contamination of the substrate with the formation of stagnant anaerobic zones in it. Moreover, it is not so much the cessation of the nitrification process itself that is dangerous, but the fact that other, heterotrophic bacteria settle in their place, and the opposite process begins in an anaerobic (oxygen-free) environment - . And at the same time, there is a high probability that it will follow the “wrong” scenarios - with the formation of such toxic compounds as hydrogen sulfide (H 2 S) and methane (CH 4), or will stop at the stage of reduction of nitrate into nitrite. And it’s good if it just ends with temporary turbidity of the water, but it can also result in a massive death of fish. A drop in filter performance (water pressure) usually indicates contamination of the substrate. If the pressure drops below 30–40% of the maximum, the filter should be washed and restarted again, otherwise this may lead to its biological “tipping over”. You should also not turn off the filter for a long time. A heavily contaminated filter can pose a danger to aquarium inhabitants after just 2–3 hours of inactivity; less polluted ones can relatively easily survive without an influx of fresh water for several hours or even days. In addition to the degree of filter contamination, this depends on many factors, including. the volume of the aquarium (in large aquariums the biochemistry is much more stable) and the density of fish stocking. But it’s better not to risk it again.
"Bacterial turbidity"
This term usually refers to the cloudiness or whitening of water, often accompanied by a musty odor. This phenomenon is typical for aquariums with undeveloped or impaired biofiltration, or occurs from an excess of organic matter (due to overfeeding or overcrowding). It can also be caused by a large infusion of fresh water or the use of “aquachemistry”. Another “sure” way to get severe cloudiness in the water is to pour a certain dose of alcohol or vodka into the aquarium (but more on that later). “Bacterial” or, as it is also called, “ciliate” turbidity is a manifestation of rapid reproduction in aquarium water of single-celled organisms, most of them heterotrophic, and their competition with each other. Samvel Kupalyan aptly called these processes “internecine wars” of microorganisms. In itself, “bacterial turbidity” is not dangerous for fish, although it is indirectly a sign of an unfavorable situation in the aquarium and is outwardly an unsightly sight. But there is no direct relationship between the turbidity of the water and the content of harmful substances in the aquarium. Toxic nitrogen compounds dissolved in water, resulting from the absence or disruption of biofiltration, are invisible to the eye. It is possible that the water has become cloudy, and tests for ammonia and nitrite show zero or very low concentrations of these substances. But it can also be the other way around: the water in the aquarium is crystal clear, but the nitrites are off the charts.
In a newly started aquarium, the water may become very cloudy on the second or third day (a textbook case, but not at all an obligatory one). If the system is properly equipped and maintained, then after a few days the “bacterial turbidity” disappears by itself. When cloudiness occurs in a mature aquarium, it goes away once the cause is eliminated. And the first thing I recommend doing if you find that the water has become cloudy “for no reason” is to increase aeration. A UV sterilizer is often recommended as a radical way to eliminate bacterial turbidity. When passing through ultraviolet radiation, the microorganisms that create turbidity die, and the water becomes clear. However, it should be understood that this does not eliminate the root cause of turbidity.
A little history
If you open old books, you will read a lot of interesting and correct things about such chemical indicators of water as PH, electrical conductivity and hardness, as well as ingenious ways to measure them, but you will not find a word about biofilters or the nitrogen cycle. The first simply did not exist then, while the second was characterized by a certain abstract concept of “biological equilibrium”, which occurs by itself a few weeks after the launch of the aquarium. The species composition of fish - mainly "livebearers" and "characinkas" - and the presence of a large number of living plants in aquariums largely compensated for these gaps in theoretical knowledge and technical equipment. I think that our country’s serious lag in the field of cichlid breeding was to a certain extent due to these “features of the national aquarium industry.” After all, keeping cichlids places increased demands on filtration. More recent publications by our reputable aquarists recognize the need for aquarium filters. " Modern aquarium science answers this question positively,” declares Igor Ivanovich Vanyushin in the article “Is filtration needed in an aquarium?” published in 1999 in the magazine “Million Friends?” At the same time, many manuals on making homemade internal filters from a plastic bottle appeared in the literature etc. “It’s impossible to imagine how many filter designs there are,” writes Igor Ivanovich, “moreover, almost every aquarist makes his contribution by changing what he saw or creating something original, his own, at the time when he becomes seriously interested in water filtration ".
At the same time, there was an understanding of the need for regular water changes in order to eliminate nitrates and other harmful substances from it. It was an amazing time of insight when aquarists discovered the reasons for the unexplained deaths and illnesses of their pets. The first domestic biofilters appeared back in the 1980s, and sometimes had very intricate designs. The disadvantages of homemade external filters, which largely negated their advantages, were bulkiness and unreliability. Therefore, the attitude towards external filters, even despite the appearance of ergonomic and reliable imported canister filters on sale, was still wary. Opinion of I.I. Vanyushin (article “We Buy Aquariums” in the magazine “Million Friends” No. 1.2000) is quite indicative in this regard: " Without going into details, all filters can be divided into external and internal. Which one is better - judge for yourself, if they perform their main task of cleaning from debris and harmful impurities in approximately the same way... The external filter almost does not limit the internal space of the aquarium. Only two tubes pass inside - for the pumping and injection lines, everything else is outside. This, perhaps, is the extent of its merits. " . A serious contraindication against branded canisters was their high cost, and Chinese ones were much inferior to them in quality. Therefore, many amateur aquarists preferred glass-type internal filters, which were widely used in our country in the late 1990s, combining stylish design and affordable price, while clearly exaggerating their biofiltration capabilities, and built-in biofilters became widespread in professional aquarium keeping. . Another alternative and a unique stage in the development of biofiltration technology in Russia was the so-called airlift, consisting of a large sponge, a lifting pipe and an aerator. This type of filter is now successfully used in nursery aquariums.
Story by Evgeniy Tsigelnitsky: “I will never forget my first filter - a split-in-half, cracked box, half a glass in size, made of pink and white “marbled” plastic (the cheapest soap dishes were made from this) on a gray, foul-smelling suction cup, thinly filled with some threads, after a week, they became completely similar to snot. A simple airlift made of a pair of glass tubes, thicker and thinner, was attached to the top of it through a crappy rubber adapter. And the threads stuffed into the filter “we are smart” (with my father) were replaced with a washcloth for dishes. I remember that filter too , and my childish amazement at how much this small, wretched contraption collected all sorts of dirt in a week in my modest, clean-looking three-bucket aquarium (completely loading a whole crackling compressor).Advanced people then used airlifts from the fraternal GDR, I also brought one after a long whining, my grandfather came all the way from Leningrad. And I became advanced - with an unreal, killer GDR sponge in a three-bucket and a marble "soap dish" that moved into a bucket ("school") boy's room. There were two whole filters - wow! By the way, this sponge worked, served, and roamed around aquariums for almost fifteen years. I still regret that it disappeared somewhere... That is, people filtered water in aquariums back in the Soviet era, but the majority of filterers did not make a cult out of this, and in no way cared about the biochemical events occurring during this... I collected the dirt - and OK…"
Denitrification and denitrifying bacteria
The process of reducing nitrates to gaseous oxides and molecular nitrogen is called denitrification. This is the second part of the nitrogen cycle. This process is used extremely rarely in freshwater aquarium keeping, but nevertheless certainly deserves consideration. Unlike nitrification, where the most important role is played by oxygen dissolved in water, denitrification processes occur in an environment devoid of oxygen, or, in scientific terms, anaerobic. Denitrification is defined as the conversion of nitrate into nitrogen, a harmless gas that bubbles out. Between the initial product (nitrate) and the final product (nitrogen gas), there are three intermediate products: in the order of their occurrence, they are nitrite (NO2), nitric oxide (NO) and nitrous oxide (N2O). That is, denitrification (like nitrification) is a multi-stage process, and its intermediate products, and in particular nitrite, are toxic. If denitrification does not occur completely, the water quality becomes much worse for fish than before this process. There are two other processes that can occur in an aquarium. These are dissimilative and assimilative absorption of nitrate. Both of them are dangerous because... produce ammonium. In fact, this is the exact opposite of nitrification - nitrate is reduced to nitrite, which is then reduced to hydroxylamine (NH 2 OH) and then ammonium.
Bacteria are responsible for all these transformations. An important difference between nitrification and denitrification is the types of bacteria that are involved in these processes. Nitrification is carried out by so-called autotrophic bacteria. This means that they obtain the carbon they need to grow from inorganic substances, particularly carbon dioxide. Denitrifying bacteria ( Bacillus, Denitrobacillas, Micrococcus, Pseudomonas etc.) are heterotrophic, that is, they receive carbon from organic sources such as sucrose, glucose, alcohols, organic acids, amino acids, etc. (there is, however, a special type of denitrator filter in which sulfur bacteria are used to process nitrate into nitrogen - autotrophs, see part 3 of the article). The essence of the beneficial effect of denitrifying bacteria is that under anaerobic conditions, i.e. extremely oxygen-poor environment, they extract the oxygen necessary for respiration from nitrate, while reducing it. Denitrifying bacteria are anaerobic bacteria. Although, to be completely correct, there are bacteria that are facultative anaerobes and, depending on the oxygen content in the environment, are able to draw it both from the outside and extract it from nitrates (therefore, by the way, it is believed that the adaptation of denitrifying bacteria to anaerobic conditions is secondary origin). But in general, as Martin Sander writes about this, “we can assume that oxygen prevents denitrification.”
Thus, for the successful denitrification process, three conditions must be met: the presence of nitrates in the aquarium, an oxygen-poor environment and the presence of organic carbon-containing substances. Carbon is used by bacteria as the main nutrient, while the need for oxygen is satisfied by nitrate. The fourth condition, which will be discussed later, is a sufficiently low redox (or as it is usually called, redox) potential.
The denitrification reaction in its classical form can be expressed by the equations:
first stage 3NO 3 - + CH 3 OH = 3NO 2 - + CO 2 + 2H 2 O and
second stage 2NO 2 - + CH 3 OH = N 2 + CO 2 + H 2 O + 2OH -.
As can be seen from the equations, nitrates are not immediately transformed into nitrogen gas, but toxic nitrites are formed first. It is only in the second stage that nitrogen is removed from the cycle by forming nitrogen gas. Ensuring that these processes occur in a controlled manner is not an easy task. In any case, this is much more difficult than establishing the biological conversion of ammonia into nitrate. In addition, there are many misconceptions associated with denitrification, including those coming from aquarists who use these technologies in practice. The process of denitrification does not always occur smoothly. Along with the beneficial effect of removing nitrates, during denitrification processes other extremely harmful substances can be formed - methane (CH 4) and hydrogen sulfide (H 2 S), since, along with denitrifying bacteria, other types of microorganisms, in particular methane-forming archaea, are involved in anaerobic processes and sulfate-reducing bacteria, also anaerobes. In particular, this happens when there is a lack of nitrates or a very low redox potential. Then the anaerobic microflora begins to satisfy the need for oxygen at the expense of other oxygen-containing chemical compounds - with the release of hydrogen sulfide and methane, both gases are toxic. Methane-forming bacteria can synthesize methane using carbon dioxide (CO 2) oxidation reactions as energy. In natural reservoirs, methane is one of the end products of the decomposition of organic matter in the bottom anaerobic zone and is formed by a highly specialized group of strict anaerobes - methane-forming archaea. Sulfate-reducing bacteria take oxygen from sulfates (SO 4 2-). This process, called desulfurization, produces hydrogen sulfide, which is known for its rotten egg smell. Also mentioned above are the bioprocesses of dissimilative and assimilative reduction of nitrates to hydroxylamine (NH 2 OH) and then ammonium. Various bacteria, as well as some actinomycetes and fungi, have the ability to do this. It is clear that all these processes, which also occur in nature, are difficult to control in an aquarium, stimulating only beneficial processes and preventing harmful processes.
Now a few words about the oxidation-reduction potential - a measure of the ability of a chemical substance to add electrons (to be reduced). Its value determines the balance between reduction and oxidation reactions in water. Another name is redox potential (from the English redox - reduction-oxidation reaction). This indicator is related to the level of contamination of the aquarium water with organic substances, as well as the age of the aquarium. A newly started aquarium is usually characterized by high redox potential values, then, as the aquarium ages, its redox potential decreases. You can maintain the redox potential at a certain level by regularly maintaining the aquarium, cleaning the soil, changing water, etc. A high positive redox potential (in a normal aquarium it is 200 – 400 mV (minivolt)) indicates the dominance of oxidative reactions over reduction reactions. A negative redox potential indicates a lack of oxygen in the water, which is lethal to most invertebrates. But for the normal course of the denitrification process, the redox potential must be negative and kept within the range of approximately -50 to -250 mV. Thus, the denitrification reaction cannot occur directly in aquarium water, but requires special anaerobic zones, which can be formed, for example, in the soil or filter. If the redox potential is higher than -50 mV (but less than zero), then the denitrification process will most likely stop at the stage of nitrite formation. And if it drops below -300 mV, then bacteria will take on sulfates.
The next problem is the availability of sufficient organic carbon required by this type of bacteria. The organic substances in the aquarium are not enough to support the process, so additional additions are required. In the above equations, methanol appears as an organic substance, but in practice, methyl alcohol is poison. The concept of a classic carbon nitrate reducer involves the use of lactose. Another option is ethyl alcohol or vodka. By the way, several years ago the idea of starting denitrification by adding vodka to the aquarium was very popular. True, not many people dared to do this, but they actively discussed it. In fact, as Dieter Brockmann writes about it, this technology has nothing to do with denitrification, that is, the breakdown of nitrates for bacterial respiration, but is rather closer to assimilation and biomass production. "With alcohol, in contrast to denitrifying filters, we stimulate primarily aerobic bacteria and only then anaerobic bacteria, which play a less important role. Assimilation means the assimilation of nitrates and phosphates, for example, by algae. The latter use both substances to obtain nitrogen and phosphorus necessary for their own metabolism and, accordingly, life support and growth. From this it follows that the increased growth of algae affects the decrease in the concentration of phosphates and nitrates in the aquarium. Previously, this effect was used in algae filters to reduce nitrates. By adding vodka to the aquarium, we thereby support assimilation , stimulating, however, not algae, but bacteria. We provide them with a source of easily processed food - ethanol in vodka. By increasing biomass, the level of phosphates and nitrates in aquarium water decreases. However, practice has shown that denitrification can only occur in a substrate containing anaerobic zones. And we also need to take care of its availability."
And further. It is necessary to balance the system so that denitrification intermediate products do not accumulate. As mentioned above, when processing nitrate, nitrite is first produced, it is toxic and should not accumulate in the aquarium. The danger of increasing nitrite concentration is one of the weak points of denitrifying systems. Well, to really thicken the picture, we must remind you that in addition to nitrates in the aquarium there are also phosphates and probably many more different substances and compounds that cannot be controlled using a standard set of aquarium tests, which is why discus fish in an aquarium cycled with the help of a denitrator suddenly become lethargic and refuse to spawn. By selectively eliminating nitrates, we only achieve the appearance of creating a closed-cycle system.
However, all this does not mean that denitrification is, in principle, inaccessible or does not make sense for aquarium hobby. Nitrate filters have been used successfully in marine aquariums for many years and are now being introduced for service in freshwater aquariums. But most of all, one hopes that denitrification is still a promising area for research, and the last word on this issue has not yet been said.
Literature
Anikshtein S. Nitrates – so harmful and so useful.
Anikshtein S. Do not neglect aeration.
Bailey M. Burgess P. The Aquarist's Golden Book.
Bersenev A. The mystery of the biofilter.
Brockman D. Nitrates.
Vanyushin I.I. Does an aquarium need filtration?
Vanyushin I.I. We buy aquariums.
Goryushkin S. Reverse osmosis in an aquarium filtration system.
Goryushkin S. Filtration and discus.
Gusev M.V., Mineeva L.A. Microbiology.
Dubinovsky M. et al. Water in the aquarium.
Dubinovsky M. et al. Filtration in the aquarium.
Dubinovsky M. Launching an aquarium.
Various information materials on marine aquarium keeping.
Kubasov A.A. Zeolites are boiling stones.
Kovalev V. Is there something wrong in the aquarium??? Let's try to figure it out!
Kovalev V. Five very important parameters of water quality and how to use them without getting confused.
Kovalev V. Composition of aquarium water: main problems.
Kuskov V. How to create and maintain biological balance.
Sander M. Technical equipment of an aquarium.
Serga T. Nitrospira – nitrite-oxidizing bacteria in aquariums.
Spiridonov M. Zeolite in the aquarium. Benefit or harm?
Telegin A. Design of open filters.
Success with a nitrate filter. Per. A.I. Goryushkina.
Frolov Yu., Yudakov V. Fundamentals of biological filtration.
Khakhinov V.V. and others. Hydrochemistry of extreme water systems with the basics of hydrobiology.
Hovanek T. What is denitrification?
Khomchenko I.G. and others. Modern aquarium and chemistry.
Tsigelnitsky E. Phytofiltration.
Sheremetyev I. Irrigated filter for an aquarium.
Elbakyan V. Nitrate horror.
Yudakov V. Brief basics of aquarium filtration.
Yartsev V. Notes on bioballs.
Yartsev V. Filters with irrigation (sump).
Brockmann D. Fische und Korallen im Meer und im Aquarium.
Holmes-Farley R. Chemistry and the aquarium: Nitrate in the reef aquarium.
Foster S. Exclusive: Hagen announces launch of Fluval G filter.
© E. Granovsky, 2009-2010
Back in 1870, Schloesing and Miintz proved that nitrification is biological in nature. To do this, they added chloroform to the wastewater. As a result, ammonia oxidation stopped. However, specific microorganisms causing this process were isolated only by Winogradsky. He also showed that chemoautotrophic nitrifiers can be divided into bacteria that carry out the first phase of this process, namely the oxidation of ammonium to nitrous acid (NH4+->N02-), and bacteria of the second phase of nitrification, converting nitrous acid into nitric acid (N02-- >-N03-). Both microorganisms are gram-negative. They belong to the Nitrobacteriaceae family.
Bacteria of the first phase of nitrification are represented by four genera: Nitrosomonas, Nitrosocystis, Nitrosolobus and Nitrosospira. Of these, the most studied species is Nitrosomonas europaea, although obtaining pure cultures of these microorganisms, as well as other nitrifying chemoautotrophs, still remains quite difficult. N. europaea cells are usually oval (0.6 -1.0 X 0.9-2.0 µm) and reproduce by binary fission. During the development of cultures in a liquid medium, mobile forms with one or more flagella and immobile zooglea are observed.
In Nitrosocystis oceanus, the cells are round, with a diameter of 1.8-2.2 microns, but they can also be larger (up to 10 microns). Capable of movement due to the presence of one flagellum or a bundle of flagella. They form zooglea and cysts.
The dimensions of Nitrosolobus multiformis are 1.0-1.5 X 1.0-2.5 microns. The shape of these bacteria is not entirely correct, since the cells are divided into compartments, lobules (-lobus, hence the name Nitrosolobus), which are formed as a result of growth inside the cytoplasmic membrane.
In Nitrosospira briensis, the cells are rod-shaped and convoluted (0.8-1.0 X 1.5-2.5 µm) and have from one to six flagella.
Among the bacteria of the second phase of nitrification, three genera are distinguished: Nitrobacter, Nitrospina and Nitrococcus.
Most of the studies were carried out with different strains of Nitrobacter, many of which can be classified as Nitrobacter winogradskyi, although other species have also been described. Bacteria have predominantly pear-shaped cells. As shown by G. A. Zavarzin, Nitrobacter reproduction occurs by budding, and the daughter cell is usually mobile, since it is equipped with one laterally located flagellum. The similarity of Nitrobacter with budding bacteria of the genus Hyphomicrobium in the composition of fatty acids included in lipids is also noted.
Data regarding nitrifying bacteria such as Nitrospina gracilis and Nitrococcus mobilis are still very limited. According to available descriptions, N. gracilis cells are rod-shaped (0.3-0.4 X 2.7-6.5 µm), but spherical shapes have also been found. Bacteria are immobile. In contrast, N. mobilis is motile. Its cells are round, about 1.5 microns in diameter, with one or two flagella.
Based on the cell structure, the studied nitrifying bacteria are similar to other gram-negative microorganisms. Some species have developed systems of internal membranes that form a stack in the center of the cell (Nitrosocystis oceanus), or are located along the periphery parallel to the cytoplasmic membrane (Nitrosomonas europaea), or form a cup-like structure of several layers (Nitrobacter winogradskyi). Apparently, enzymes involved in the oxidation of specific substrates by nitrifiers are associated with these formations.
Nitrifying bacteria grow on simple mineral media containing an oxidizable substrate in the form of ammonium or nitrites and carbon dioxide. In addition to ammonium, hydroxylamine and nitrites can be a source of nitrogen in construction processes.
Nitrobacter and Nitrosomonas europaea have also been shown to reduce nitrite to form ammonium.
A microorganism such as Nitrosocystis oceanus, isolated from the Atlantic Ocean, is an obligate halophile and grows on a medium containing seawater. The pH range at which growth of different species and strains of nitrifying bacteria is observed is 6.0-8.6, and the optimal pH value is most often 7.0-7.5. Among Nitrosomonas europaea, strains are known that have a temperature optimum at 26 or about 40 °C, and strains that grow quite quickly at 4 °C.
All known nitrifying bacteria are obligate aerobes. They need oxygen for the oxidation of ammonium into nitrous acid:
and for the oxidation of nitrous acid into nitric acid:
But the entire process of converting ammonium into nitrates occurs in several stages with the formation of compounds where nitrogen has different degrees of oxidation.
The first product of ammonium oxidation is hydroxylamine, which is possibly formed as a result of the direct inclusion of molecular oxygen in NH+4:
However, the mechanism of ammonium oxidation to hydroxylamine has not been fully elucidated. Conversion of hydroxylamine to nitrite:
is believed to occur through the formation of hyponitrite NOH, as well as nitric oxide (NO). As for nitrous oxide (N2O), found during the oxidation of ammonium and hydroxylamine by Nitrosomonas europaea, most researchers consider it to be a byproduct formed mainly from the reduction of nitrite.
A study of Nitrobacter oxidation of nitrite using the heavy isotope of oxygen (18O) in experiments showed that the resulting nitrates contain significantly more 18O when the labeled substance is water rather than molecular oxygen. Therefore, it is assumed that the NO2-H2O complex is formed first, which is then oxidized to NO2-. In this case, electrons are transferred through intermediate acceptors to oxygen. The entire nitrification process can be represented in the form of the following diagram (Fig. 137), the individual stages of which, however, require clarification.

In addition to the first reaction, namely the formation of hydroxylamine from ammonium, subsequent stages provide organisms with energy in the form of adenosine triphosphate (ATP). ATP synthesis is associated with the functioning of redox systems that transfer electrons to oxygen, similar to what occurs in heterotrophic aerobic organisms. But since the substrates oxidized by nitrifiers have high redox potentials, they cannot interact with nicotinamide adenine dinucleotides (NAD or NADP, E1/0 = -0.320 V), as happens during the oxidation of most organic compounds. Thus, the transfer of electrons to the respiratory chain from hydroxylamine apparently occurs at the level of flavin:
When nitrite is oxidized, the inclusion of its electrons in the chain probably occurs at the level of either cytochrome type c or cytochrome type a. In connection with this feature, the so-called reverse, or inverted, electron transport is of great importance in nitrifying bacteria, which occurs with the expenditure of energy from part of ATP or the transmembrane potential formed during the transfer of electrons to oxygen (Fig. 138).

In this way, chemoautotrophic nitrifying bacteria are provided with not only ATP, but also NADH, necessary for the absorption of carbon dioxide and for other constructive processes.
According to calculations, the efficiency of free energy use by Nitrobacter can be 6.0-50.0%, and Nitrosomonas - even more.
The assimilation of carbon dioxide occurs mainly as a result of the functioning of the pentoeophosphate carbon reduction cycle, otherwise called the Calvin cycle (see Fig. 134).

The result is expressed by the following equation:
where (CH2O) means the resulting organic substances having a level of carbon reduction. However, in reality, as a result of the assimilation of carbon dioxide through the Calvin cycle and other reactions, primarily through the carboxylation of phosphoenolpyruvate, not only carbohydrates are formed, but also all other cellular components - proteins, nucleic acids, lipids, etc. It has also been shown that Nitrococcus mobilis and Nitrobacter winogradskyi can produce poly-β-hydroxybutyrate and glycogen-like polysaccharide as storage products. The same compound was found in Nitrosolobus multiformis cells. In addition to carbon-containing reserve substances, nitrifying bacteria are capable of accumulating polyphosphates, which are part of metachromatic granules.
Even in his first works with the nitrifier, Vinogradsky noted that the presence of organic substances in the environment, such as peptone, glucose, urea, glycerin, etc., is unfavorable for their growth. The negative effect of organic substances on chemoautotrophic nitrifying bacteria was repeatedly noted in the future. There was even an opinion that these microorganisms are not at all capable of using exogenous organic compounds. Therefore, they came to be called “obligate autotrophs.” However, recently it has been shown that these bacteria are capable of using some organic compounds, but their capabilities are limited. Thus, a stimulating effect on the growth of Nitrobacter was noted in the presence of nitrite from yeast autolysate, pyridoxine, glutamate and serine, if they are added to the medium in low concentrations. The inclusion of pyruvate, α-ketoglutarate, glutamate and aspartate in proteins and other components of Nitrobacter 14C cells has also been shown. It is also known that Nitrobacter slowly oxidizes formate. The incorporation of 14C from acetate, pyruvate, succinate and some amino acids, mainly into the protein fraction, was found when these substrates were added to Nitrosomonas europaea cell suspensions. Limited assimilation of glucose, pyruvate, glutamate and alanine has been established for Nitrosocystis oceanus. There is evidence of the use of 14C-acetate by Nitrosolobus multiformis.
It has also recently been established that some strains of Nitrobacter grow on a medium with acetate and yeast autolysate not only in the presence, but also in the absence of nitrite, although slowly. In the presence of nitrite, the oxidation of acetate is suppressed, but the incorporation of its carbon into various amino acids, proteins and other cellular components is increased. Finally, there is evidence that the growth of Nitrosomonas and Nitrobacter is possible on a medium with glucose under the analyzed conditions, which ensure the removal of products of its metabolism that have an inhibitory effect on these microorganisms. Based on this, a conclusion is made about the ability of nitrifying bacteria to switch to a heterotrophic lifestyle. However, more experiments are needed to draw final conclusions. It is important, first of all, to find out how long nitrifying bacteria can grow under heterotrophic conditions in the absence of specific oxidizable substrates.
Chemoautotrophic nitrifying bacteria are widespread in nature and are found both in soil and in various bodies of water. The processes they carry out can occur on a very large scale and are of significant importance in the nitrogen cycle in nature. Previously, it was believed that the activity of nitrifiers always contributes to soil fertility, since they convert ammonium into nitrates, which are easily absorbed by plants, and also increase the solubility of certain minerals. Now, however, views on the importance of nitrification have changed somewhat. Firstly, it has been shown that plants absorb ammonium nitrogen and ammonium ions are better retained in the soil than nitrates. Secondly, the formation of nitrates sometimes leads to undesirable acidification of the environment. Thirdly, nitrates can be reduced by denitrification to N2, which leads to soil depletion of nitrogen.
It should also be noted that, along with nitrifying chemoautotrophic bacteria, heterotrophic microorganisms are known that are capable of carrying out similar processes. Heterotrophic nitrifiers include some fungi from the genus Fusarium and bacteria of such genera as Alcaligenes, Corynebacterium, Achromobacter, Pseudomonas, Arthrobacter, Nocardia.
It has been shown that Arthrobacter sp. oxidizes ammonium in the presence of organic substrates to form hydroxylamine and then nitrites and nitrates. In addition, hydroxamic acid may be formed. A number of bacteria have been shown to carry out nitrification of organic nitrogen-containing compounds: amides, amines, oximes, hydroxamates, nitro compounds, etc. The ways of their transformation are presented as follows:

The extent of heterotrophic nitrification in some cases can be quite large. In addition, this produces some products that have toxic, carcinogenic, mutagenic effects and compounds with a chemotherapeutic effect. Therefore, considerable attention is now being paid to the study of this process and elucidation of its significance for heterotrophic microorganisms.
Life of plants: in 6 volumes. - M.: Enlightenment. Edited by A. L. Takhtadzhyan, editor-in-chief, corresponding member. USSR Academy of Sciences, prof. A.A. Fedorov. 1974 .
Convert ammonia and ammonium salts into nitric acid salts: nitrates: nitrosobacteria, nitrobacteria. Distributed in soils and water bodies... Big Encyclopedic Dictionary
Convert ammonia and ammonium salts into nitric acid salts: nitrates: nitrosobacteria, nitrobacteria. Distributed in soils and water bodies. * * * NITRIFYING BACTERIA NITRIFYING BACTERIA convert ammonia and ammonium salts into nitrogen salts... ... encyclopedic Dictionary
nitrifying bacteria- nitrifikatoriai statusas T sritis ekologija ir aplinkotyra apibrėžtis Nitritinės (Nitrosomonas genties) ir nitratinės (Nitrobacter genties) bakterijos, paverčiančios amonio druskas nitratais. atitikmenys: engl. nitrifiers; nitrifying bacteria vok… Ekologijos terminų aiškinamasis žodynas - carry out oxidation reactions of reduced nitrogen compounds. Representatives of the genus Nitrosomonas oxidize ammonia to nitrites, and bacteria of the genus Nitrobacter oxidize nitrites to nitrates. They belong to the autotrophic chemosynthesizing aerobic... ... Geological encyclopedia
Based on the type of nutrition, all organisms are divided into autotrophs and heterotrophs. Autotrophs, which means “self-feeding” in Greek, can build all the compounds of their cells from carbon dioxide and other inorganic substances. Source... ... Biological encyclopedia
Ammonia, formed in soil, manure and water during the decomposition of organic matter, is quickly oxidized to nitrous and then nitric acid. This process is called nitrification.
Until the middle of the 19th century, more precisely, before the work of L. Pasteur, the phenomenon of nitrate formation was explained as a chemical reaction of ammonia oxidation by atmospheric oxygen, and it was assumed that the soil played the role of a catalyst in this process. L. Pasteur suggested that the formation of nitrates is a microbiological process. The first experimental evidence of his hypothesis was obtained by T. Schlesing and A. Münz in 1879. The researchers passed wastewater through a long column of sand and CaCO 3 . During filtration, ammonia gradually disappeared and nitrates appeared. Heating the column or adding antiseptics stopped the oxidation of ammonia.
However, neither the mentioned researchers nor the microbiologists who continued to study nitrification were able to isolate cultures of nitrification pathogens. Only in 1890-1892. S. N. Vinogradsky, using a special technique, isolated pure cultures of nitrifiers. The scientist suggested that nitrifying bacteria do not grow on ordinary nutrient media containing organic matter, which explained the failures of his predecessors.
Indeed, nitrifiers turned out to be chemolithoautotrophs, i.e. bacteria that use the energy of oxidation of ammonia or nitrous acid to synthesize organic substances from CO 2 (chemosynthesis). Therefore, their cells are very sensitive to the presence of organic compounds in the environment. Nitrifying bacteria were isolated on mineral nutrient media.
S. N. Vinogradsky established that there are two groups of nitrifiers: one carries out the oxidation of ammonia to nitrous acid (NHJ-? N0 2) - the first phase of nitrification, the other - the oxidation of nitrous acid to nitric acid (NOj-? NOj) -
second phase of nitrification.
Representatives of both groups are classified as family Nitrobacteriaceae. These are single-celled gram-negative bacteria. Nitrifying bacteria include rod-shaped, elliptical, spherical, convoluted and lobed, pleomorphic cells. Cell sizes range from 0.3 to 1 µm in width and from 1 to 3 µm in length. There are mobile and immobile forms with polar, subpolar and peritrichal flagellation.
Nitrifying bacteria reproduce mainly by division, with the exception of Nitrobacter, which is characterized by budding. Almost all nitrifiers have a well-developed system inside cytoplasmic membranes, which vary significantly in shape and location in the cells of individual species. The membranes of the cytoplasm are similar to those of photosynthetic purple bacteria.
Bacteria of the first phase of nitrification are represented by the following genera: Nitrosomonas, Nitrosococcus, Nitrosospira, Nitrosolobus And Nitroso-vibrio. The most thoroughly studied to date Nitrosomonas europaea(Fig. 42, A). It consists of short oval rods measuring 0.8-1 x 1-2 microns. In liquid cell culture Nitrosomonas go through a number of developmental stages. The two main ones are represented by a mobile form and immobile zooglea. The motile form has a subpolar flagellum or a bundle of flagella.
Representatives of other genera of bacteria that cause the first phase of nitrification have also been described.
The second phase of nitrification is carried out by representatives of the genera Nitrobacter, Nitrospira And Nitrococcus. The largest number of studies have been conducted with Nitrobacter winogradskyi(Fig. 42, />), however, other species are also described (for example, Nitrobacter agilis). Nitrobacter cells have an elongated, wedge-shaped or pear-shaped shape, the narrower end is often bent into the beak, the cell dimensions are 0.6-0.8 x 1-2 microns. During budding, the daughter cell is usually mobile, since it has one polar flagellum. The alternation of mobile and immobile stages in the development cycle is known.
Rice. 42.
A - Nitrosomonas euro race; B - Nitrobacter winogradskyi
Other types of bacteria that cause the second phase of nitrification have also been described.
Nitrifying bacteria are cultivated on simple mineral media containing ammonia or nitrites (oxidizable substrates) and carbon dioxide (the main carbon source). The source of nitrogen for these organisms is ammonia, hydroxyl and n and nitrites.
Nitrifying bacteria develop at pH 6.0-8.6, the optimum reaction of the environment is pH 7.5-8.0. At values below pH 6 and above pH 9.2, bacteria do not develop. The optimal temperature for the development of nitrifiers is 25-30 °C. Studying the relationship of different strains Nitrosomonas europaea to temperature showed that some of them have an optimum development at 26 °C or about 40 °C, others are able to grow quite quickly at 4 °C.
Nitrifiers are obligate aerobes. Using atmospheric oxygen, they oxidize ammonia to nitrous acid (the first phase of nitrification):
Consequently, ammonia - a waste product of ammonifying bacteria - is used to produce energy Nitrosomonas, and nitrites formed during the life processes of the latter serve as a source of energy for Nitrobacter.
According to modern concepts, the nitrification process occurs on the cytoplasmic and inside the cytoplasmic membranes and takes place in several stages. The first product of ammonia oxidation is hydroxylamine, which is then converted to nitroxide (NOH) or peroxonitrite (ONOOH), the latter, in turn, is further converted to nitrite, and nitrite to nitrate. The entire nitrification process is illustrated by the following diagram:

Nitroxyl, like hydroxylamine, apparently can dimerize into hyponitrite or convert into nitrous oxide N20, a by-product of nitrification. In addition to the first reaction (formation of hydroxylamine from ammonium), all subsequent transformations are accompanied by the synthesis of high-energy bonds in the form of ATP.
Nitrifiers fix CO2 through the reductive pentosephosphagic cycle (Calvin cycle). As a result of subsequent reactions, not only carbohydrates are formed, but also other compounds important for bacteria - proteins, nucleic acids, fats, etc.
For a long time, nitrifying bacteria were classified as obligate chemolithoautotrophs. Later, data were obtained on the ability of these bacteria to use certain organic substances. Thus, a stimulating effect on growth was noted Nitrobacter nitrite, yeast autolysate, pyridoxine, glutamic acid and serine. It is believed that some nitrifying bacteria have the ability to switch from autotrophic to heterotrophic nutrition. However, nitrifiers do not grow on conventional nutrient media, since the large amount of easily digestible organic substances contained in such media retards their development. However, in nature, such bacteria develop well in chernozems, manure, composts, etc. in places where there is a lot of organic matter.
This contradiction turns out to be insignificant if we compare the amount of easily oxidizable carbon in the soil with the concentrations of organic matter that nitrifiers must withstand in crops. Thus, soil organic matter is represented mainly by humic substances, which account for 71-91% of the total carbon in chernozem, and easily digestible water-soluble organic substances account for no more than 0.1% of the total carbon. Consequently, nitrifiers do not encounter large quantities of easily digestible organic matter in the soil.
Nitrate accumulation occurs at different rates in different soils. The richer the soil, the more nitric acid compounds it can accumulate. There is a method for determining the nitrogen available to plants in the soil based on the nitrification capacity. Therefore, the intensity of nitrification can be used to characterize the agronomic properties of the soil.
At the same time, during nitrification, only the conversion of one plant nutrient - ammonia - into another form - nitric acid. Nitrates, however, have some undesirable properties. While the ammonium ion is absorbed by the soil, nitric acid salts are easily washed out of it. In addition, nitrates are reduced as a result of denitrification to N 2, which also depletes the nitrogen reserves of the soil. All of the above significantly reduces the utilization rate of nitrates by plants.
In a plant organism, nitric acid salts must be reduced before being included in synthesis, which requires energy. Ammonium is used directly. In this regard, scientists raised the question of the possibility of artificially reducing the intensity of nitrification using specific inhibitors that suppress the activity of nitrifying bacteria and are harmless to other organisms. Numerous industrial preparations of nitrification inhibitors (2-chloro-6-(trichloromethyl)-pyridine, nitropyrine, etc.) synthesized on a pyridine basis have already been proposed. Nitrification inhibitors suppress only the first phase of nitrification and do not affect the second, as well as heterotrophic nitrification. When using nitrification inhibitors (nitropyrine), the efficiency of nitrogen fertilizers increases from 50 to 80%.
""sb Heterotrophic nitrification. Some heterotrophic microorganisms are also capable of nitrification. These include bacteria from the genera Pseudomonas, Arthrobacter, Corynebacteriitis, Nocardia and certain species of fungi from the genera Fusarium, Aspergillus, Penici/lium, Cladosporium. Determined that Arthrobacter sp. in the presence of organic substrates causes the oxidation of ammonia to form hydroxylamine, and then nitrite and nitrate. Some bacteria cause nitrification of nitrogen-containing organic substances such as amides, amines, hydroxamic acids, nitro compounds (aliphatic and aromatic), oximes, etc. However, it is believed that heterotrophic nitrification does not serve as a source of energy for the listed organisms.
Heterotrophic nitrification occurs in natural conditions (soils, reservoirs and other substrates). It can acquire dominant importance, especially in atypical conditions (for example, with a high content of organic C- and N-compounds in alkaline soil, etc.). Heterotrophic microorganisms not only promote nitrogen oxidation under such conditions, but also cause the formation and accumulation of toxic substances, compounds with carcinogenic and mutagenic, as well as chemotherapeutic effects. Due to the fact that some of the listed compounds are harmful to humans and animals even in relatively low concentrations, the possibility of their formation in nature is being carefully studied.
- In recent years, the ability of bacteria to anaerobically oxidize ammonia has been discovered. This process, called anammox (An-attox), plays an important role in wastewater treatment. The bacteria that carry it out belong to the group of planctomists. (Note: re